24
24why?????????????????
ans is o dear which was also stated by subash..........
 21
21i know its debatable and not easily swallowable........
lets c if i get a chance i'll ask ma teacher n tell u wat he says...
u try n do da same
 24
24yaar.......i still stand by my first post..............Zero isomers
 21
21this is a bridge-head situation..... so i feel free rotation will b retarded....
ur point is correct that in biphenyl situation planarity is due to double bond thing.....
but ther 2 phenyls r connectged by a c-c bond......
here to v jus hav 1 carbon sufficing both the rings.......... so i m not sure!!!
 24
24i know what u r saying is similar to biphenyl situation...........but see there too there was planarity concept used due to partial double bond character due to resonance[1]
 24
24hmmmmmmmmmm[12][12].............I dont think it will be possible......maybe i am wrong...........but there is no double bond between those 2 rings....so both rings will be free to rotate....so i dont think then we can say 2 isomers..........we have to say then infinite isomers.....just like confirmation.....[2]
 21
21@Eureka :
ther r 2 geo isomers as the ther are two ring structure involved in the structuer
ther will b 2 geo isomers possible :
1. wen both the rings are coplanar!!!
2. wen both rings are perpendicular!!( as shown in the diag by subhash)
 1
1...but there will be two geo iso !! [12]
 1
1and b/w try naming this .
 1
1there's only one structure . so no isomers for this .plane of symmetry.
 21
21dude, i'll tell u y tom!! ( hope its fine to u)
abhi thoda isc padhna hai got che prac tom.....
c ya tom.....
 21
21ohhhhh!!!!
yeah..... o stereoiso.... 2 geometrical isomers
 24
24is there some trick involved??????????[12]
 24
24no worries when eureka is here........[3]
btw nic pic bro..........[1]
 11
11im the one sleepy[13][13][13]
got it now
 24
24how chiral??????????????????
I dont think I am sleeepy yet...........[12]
2 identical methyl groups attatched.............
 11
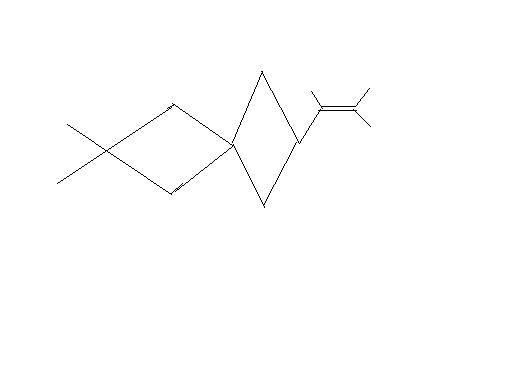
11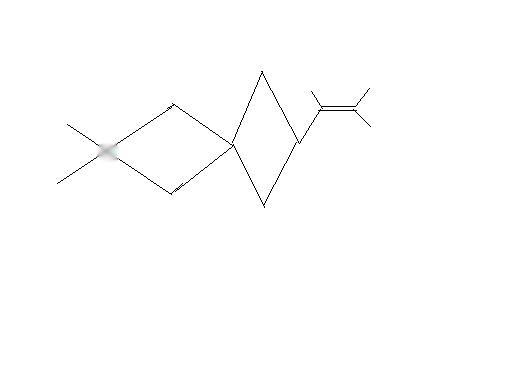
the carbon marked wont it be chiral i dont see any symmetry either
 24
24yes there is no confirmational isomerism,
no cis trans becoz three identical groups about double bond
so ans will be zero.....[1]
 11
11ans is a surprise (atleast to me) zero
 24
24i cant see any cis trans in it..........[1]........whats the ans????
 11
11i dunno i dint get it thats why posting