1) i'm not very sure but this rkn should take place in the presence of a reagent furnishing H-. the given reagent only supplies H+.
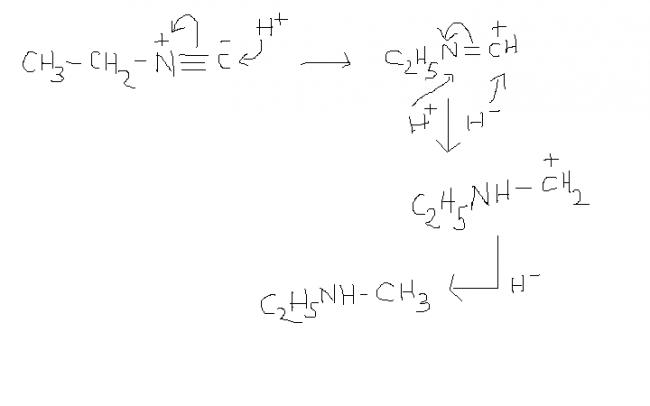
either answer all these ques..or tell me an online source from where I can learn about these all isocyanide reactions .............
1) ethyl isocyanide on reaction with Na/alcohol = ??
2) acetaldoxime reacts with phosphoruous pentoxide to give ??
3) acid hydrolsis of methyl isocyanide gives ??
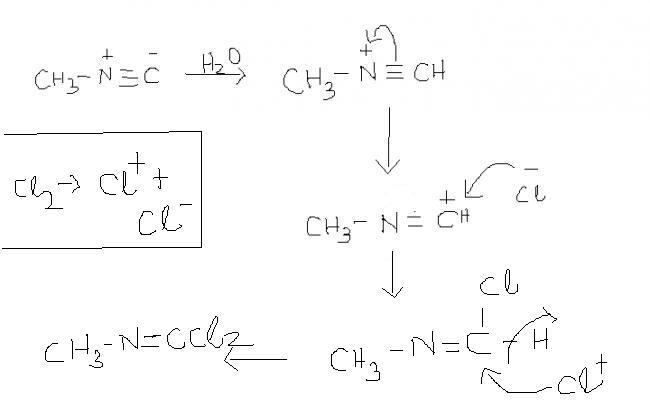
4) methyl isocyanide reacts with chlorine to give ??
1) i'm not very sure but this rkn should take place in the presence of a reagent furnishing H-. the given reagent only supplies H+.
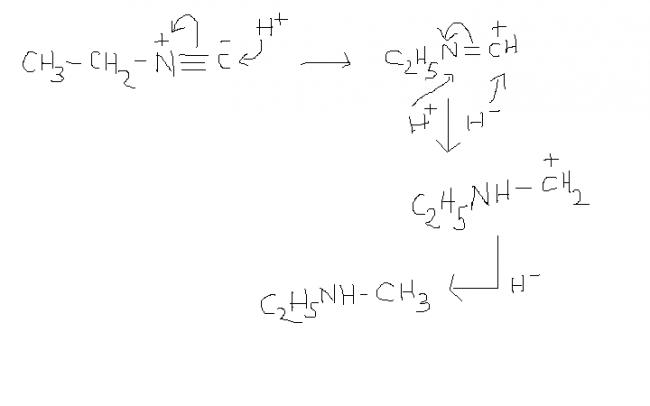
if more moles are used then, activity of Cl- will be more as ders possibilty of +ve charge on C , and also H+ also needn't be extracted out due to hindrance by two Cl groups.
also N- can't sustain another electronegative atom like Cl. thus, Cl+ would remain unreactive.
frm reagents like LiAlH4 or NaBH4 or NaH etc. anyone of reagents furnishing H- should hv been mentioned.
i strongly think so. but, if mechanism could be approached in any other way , then ?????
4) ya, right. i hv taken two moles of Cl2 , dats why its coming so. otherwise the answer u hv given is right ( with a single mole of Cl2 ).
1) and 1st reaction should take place in the presence of H- , otherwise when H+ is added to C- in the substrate , the reaction proceeds in the backward direction and again the substrate is formed ( in the absence of H- )
Ans4 a bit wrong...here is the answer....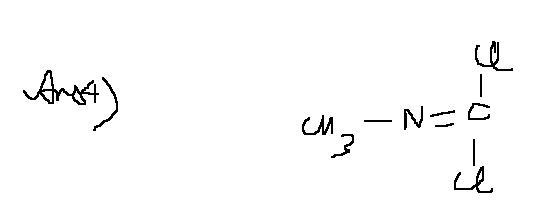
and one dbt in soln1 ......from where is that H- coming???
help hs come.
2) NOTE : keto form is more stable thn enol form. so tauto merism takes place.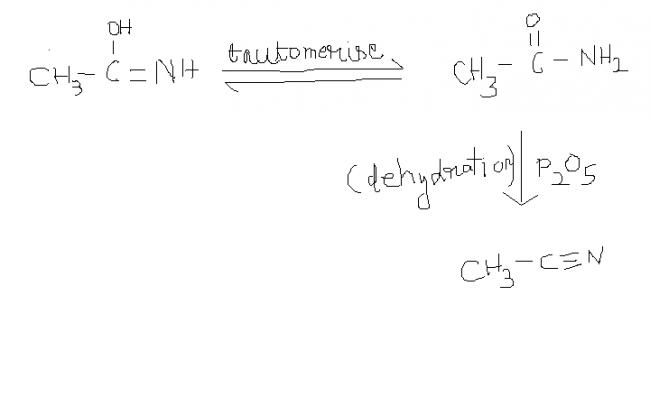
2 ) CH3-N≡ C ----(acidic hydrolysis)----> CH3-NH2 + HCOOH (Formic acid).....this is also a test 2 distinguish b/w cyanides & isocyanides ...
1 ) R-N ≡ C + Na/alcohol -------> R-NH-CH3 ..... Na/alcohol is a reducing agent....