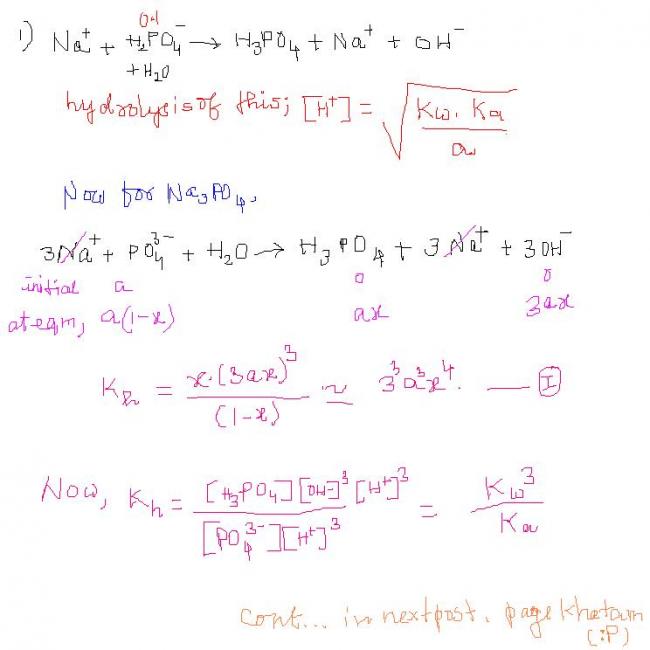
NOW for H3PO4 Ka1=10-4 Ka2=10-7 Ka3=10-10 Find the pH of: 1)mixture of NaH2PO4 (0.1M + Na3PO4(0.1M) 2)H3PO4(0.1M) +NaOH in 1L solution each
-
UP 0 DOWN 0 0 3

NOW for H3PO4 Ka1=10-4 Ka2=10-7 Ka3=10-10 Find the pH of: 1)mixture of NaH2PO4 (0.1M + Na3PO4(0.1M) 2)H3PO4(0.1M) +NaOH in 1L solution each