Ya Asish that way ya ure right
But c this is my confusion :
In isobaric w = pΔV
In adiabatic w = pΔV/ γ-1 > w due to isobaric ????
If w1 w2 w3 r the works done in isothermal , adiabatic , & isobaric processes , the correct order will be , if ΔV is same for all the processes
(A) w1 > w2 > w3
(B) w3 > w2 > w1
(C) w3 > w2 > w1
(D) w3 > w1 > w2
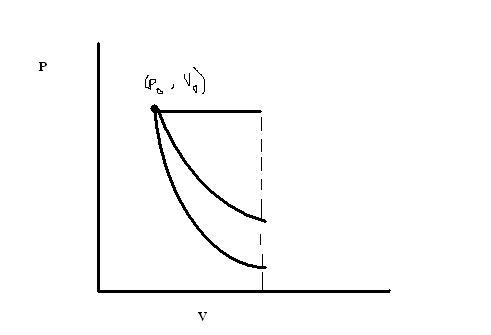
hori line - isobaric
middle line - isothermal
bottom line - adiabatic
so, w3>w1>w2
Ya Asish that way ya ure right
But c this is my confusion :
In isobaric w = pΔV
In adiabatic w = pΔV/ γ-1 > w due to isobaric ????
@uttara: the mistake ur doing is this
In adiabatic w = pΔV/ γ-1 > w due to isobaric ????
the bolded portion is wrong
W(adia) = Δ(PV)/(γ-1)
Δ(PV) ≠P(ΔV)
so ull have to go by other means