i dont think it shows geometrical isomerism...but it shows optical isomerism... in the following image replace en by gly and co by cr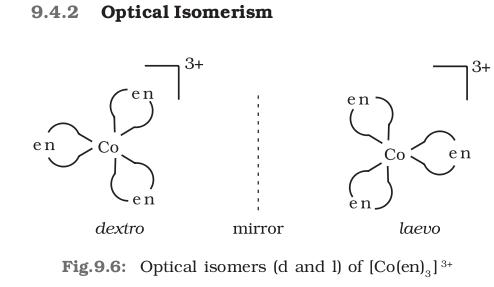
source : pg 245 class 12 chemistry ncert
can someone please explain to me how
[Cr(gly)3] shows geometrical and optical isomerism
-
UP 0 DOWN 0 0 6

6 Answers
providing some links---------------
http://www.transtutors.com/userfiles/image/ATUL/Isomerism%20Figure%207.JPG
thanks
i have understood the optical isomerism part,
but the geometrical isomerism part is still not clear..
i am posting the images given in the link by rocky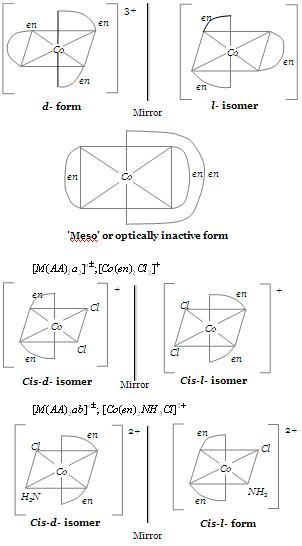
in this image the third structure
i dont think that this form exist bcoz this type of linkage is only possible in large chains and in this case it forms a five membered ring in which three groups( Co, NH2,NH2) have to be in a plane which i think will not be stable..
It does exhibit optical isomerism as shown above and it also exhibits geometrical isomerism exist in cis and trans forms since the ligand is an unsymmetrical bidentate ligand....
when atleast2 pairs of similar atoms N and O are opposite to each other at 180 degrees , it is the trans form , and when the 3 pairs of dissimilar atoms are at 180 degrees it is the cis form...
It exhibits cis and trans geometrical isomerism. In the cis form the three NH2 group of the ligand forms one face of the octahedron and the three O atoms forms the other face, whereas in the trans form the three NH2 group of the ligand form one meridian of the octahedron and the three oxygen atom forms the other head. This type of isomerism can also be called facial-meridional isomerism.