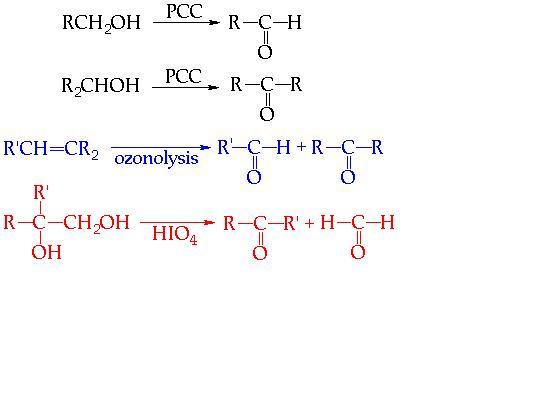
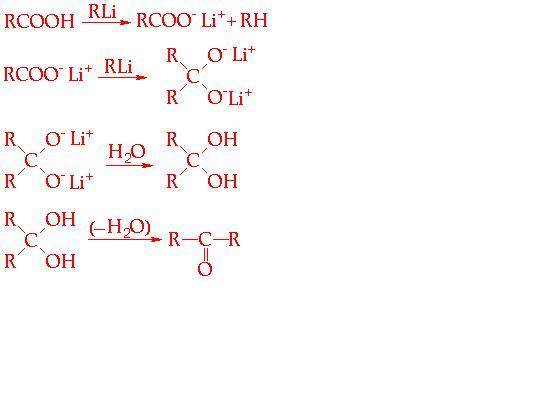
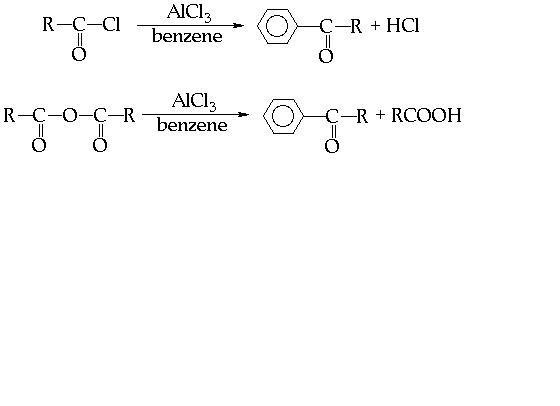
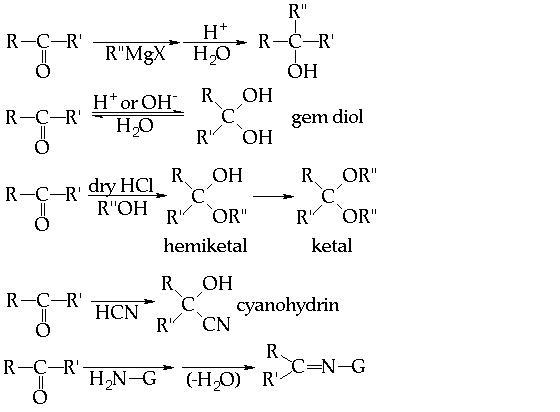
THESE R SOME WHICH I FOUND IMPORTANT.......
FROM THE VIEW OF IIT.....
19 Answers
Ask Questions
Simple, right? Wrong. Far too many students do not ask questions. You have many opportunities to clear up the unclear: during class, after class, in my office, at recitation, in lab. Take advantage of me. That's why I'm here. Ask.
My favorite questions are of the type: "I worked this problem and got a different answer than the one in the book. I thought that this functional group did that but in this case I guess it doesn't. Why?"
My least favorite questions are of the types: " I don't get chapter 6?" and "What will be on the quiz?"
Hope this info will be helpful to u all...........
The Daily Work-Out
The basketball player, the swimmer, the violinist, the dancer, the student of foreign language, all of them practice daily if they are to succeed and improve. Organic chemistry takes time and practice. It takes discipline and planning. But so does weight training.
Just do it.
Learn, Don't Memorize
Organic chemistry consists of a few basic principles and many extensions and applications of these principles. There IS rhyme and reason to organic chemistry. Relatively little memorization is required if you grasp the major concepts and develop flexibility in applying those concepts.
Some facts and fundamentals must be learned (memorized) in order that you have a working "vocabulary." So one of your main tasks is to identify the key ideas, learn them, and then apply them. Do not attempt to memorize your way through this course. It does not work. There are more than 14,000,000 organic compounds known, and you will never be able to guess which ones might be chosen as examples.
More Survival Tips
Keep Up
Falling behind is death. And once you are behind, it is even harder to catch up while staying on top of the new material. So don't dig this hole for yourself. Start right now and keep on schedule.
You cannot see how the material you cover today will be used again three chapters from now. But it will. By focusing on basis principles, you will see that the same reactions keep appearing over and over again. The specific details will change, but the principles remain the same.
If you fail to comprehend something now, you set yourself up for compounded problems later. Deal with the issue now, while it is fresh in your mind. The best way to keep up is to plan to do organic every day. Weekends included.
Your Basic Four
1 Read the Text; Think
Read the text or at the very least skim it BEFORE CLASS. This allows you to review material you might already know. You will also have an idea about what material is coming. You will know what information is in the book - and you won't have to write it all down in class. You may even be able to generate a few questions. You can pay special attention to any unclear parts during lecture.
2 Attend Lecture; Listen; Think
It is a well know fact that the people that struggle the most with this course are the ones that show up at lecture the least. Inverse correlation. Think about it.
Occasionally during class, I will ask you to pull out a piece of paper and write down a mechanism or work a problem or something. I will then collect your creations. These will not be graded, so relax. What good are they, you ask?? They allow me to take attendance rather easily. They allow me to see whether you've read the assignment for the day, where you are with the material, what's confusing and what you've got a handle on.
3 Take Notes; Think
Listen in lecture and take notes. Note that I put LISTENING first and note taking second! If you have skimmed the text, you will know what material appears in the book, such as which mechanisms are drawn out etc. That way, you have to write less. Make a note about things you did not understand then ask about it, either during class, after class, or later in my office.
Too many people develop the bad habit of trying to write down everything the professor says/writes/shows during class. This would not be bad in itself, except that many folks turn OFF their brains while doing it. They do not attempt to understand what they are writing. Then, when they attempt to use those notes later in working problems and stuyding, they have no idea what the notes mean. Solution: think more, write less, use the textbook as a backup.
Your lecture notes should not be a simple rote copying of whatever appears from my mouth or from my pen or piece of chalk. Your notes allow you to begin to process, organize, highlight, and identify concepts in a way that is useful TO YOU. Some students have found it helpful to bring ink pens of different colors to class so that they can organize the notes by color as they go. You may discover another method that works for you.
4 Work Problems; Think
Work problems, do them many times. Work lots of problems. Do the ones assigned until you understand them. Then do them again. Do the ones that weren't assigned. Generate lots of paper for the recycle bins. Problems ask you to use the material in ways other that the text or lecture can do. Problems give you the opportunity to discover which concepts and ideas are clear and unclear. Problems can be fun.
Study by working the problems, NOT only by reading your notes and the text. Your best measure of your facility with the material is whether you can work problems.
Working problems is essential to success. Work some more problems.
THESE R SOME OF THE THOUGHTS HOW TO LEARN ORG. CHEM...
You have probably heard horror stories about this class. About how organic is the hardest course, the flunk-out course, etc. You may feel overwhelmed by the number of compounds, names, reactions, and mechanisms that confront you. My suggestion to you is to read these pages and take the suggestions to heart.
Personally, I think organic chemistry is a great lot of fun. I want you to enjoy this class as much as I do, but I can't learn the material for you. Sorry.
If you are to be a successful student of organic chemistry, you must discover for yourself how to learn chemistry. Different people learn, take in, process, and integrate information, differently. What works for you will not always work for someone else. An explanation that clarifies for one person will simply add more confusion for another. Try lots of things until you find the ones that work for you.
Important Rules to Live By If You Wish to Succeed in CHEM211
1. Work the Problems
2. Don't Get Behind
You ignore these rules at your peril.
On Studying:
Organic chemistry cannot be learned the night before the exam.
Don't even try.
Learning chemistry, or any subject, involves practice. Far too many students attempt to prepare for an exam by spending hours and hours in the day and a half before the test, shoving loose pieces of random information at their heads. No wonder many folks are frustrated by their performance in the class.
One analogy that works for me is that learning organic chemistry is like learning to play the piano. (Okay, so I'm a pianist, shoot me.) You do not sit down in front of the keyboard the evening before the recital and attempt to learn the A minor 2-Part Invention by Bach. In order to play at all, you need to practice every day in the preceeding weeks and months (and years).
Organic chemistry cannot be learned the night before the exam. Don't even try. You must practice every day - read, listen, work problems, re-read, take notes, re-organize ideas, ask questions.
MECHANISM OF MICHAEL's RXn
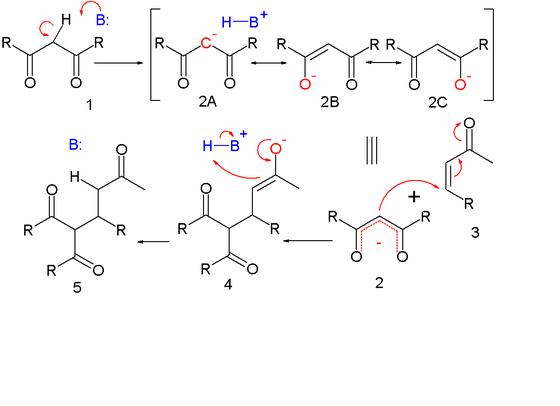
Deprotonation of 1 by base leads to carbanion 2 stabilized by its electron-withdrawing groups. Structures 2a to 2c are three resonance structures that can be drawn for this species, two of which have enolate ions. This nucleophile reacts with the electrophilic alkene 3 to form 4 in a conjugate addition reaction. Proton abstraction from protonated base (or solvent) by the enolate 4 to 5 is the final step.
The course of the reaction is dominated by orbital, rather than electrostatic, considerations. The HOMO of stabilized enolates has a large coefficient on the central carbon atom while the LUMO of many alpha, beta unsaturated carbonyl compounds has a large coefficient on the beta carbon. Thus, both reactants can be considered soft. These polarized frontier orbitals are of similar energy, and react efficiently to form a new carbon-carbon bond.
Like the aldol addition, the Michael reaction may proceed via an enol, silyl enol ether in the Mukaiyama-Michael addition, or more usually, enolate nucleophile. In the latter case, the stabilized carbonyl compound is deprotonated with a strong base (hard enolization) or with a Lewis acid and a weak base (soft enolization). The resulting enolate attacks the activated olefin with 1,4-regioselectivity, forming a carbon-carbon bond. This also transfers the enolate to the electrophile. Since the electrophile is much less acidic than the nucleophile, rapid proton transfer usually transfers the enolate back to the nucleophile if the product is enolizable; however, one may take advantage of the new locus of nucleophilicity if a suitable electrophile is pendant. Depending on the relative acidities of the nucleophile and product, the reaction may be catalytic in base. In most cases, the reaction is irreversible at low temperature, due to least-motion arguments.
Michael reaction The Michael reaction or Michael addition is the nucleophilic addition of a carbanion to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds. Many asymmetric variants exist.  In this scheme the R and R' substituents on the nucleophile are electron-withdrawing groups such as acyl and cyano making the methylene hydrogen acidic forming the carbanion on reaction with base B:. The substituent on the activated alkene is usually a ketone making it an enone but it can also be a nitro group.
In this scheme the R and R' substituents on the nucleophile are electron-withdrawing groups such as acyl and cyano making the methylene hydrogen acidic forming the carbanion on reaction with base B:. The substituent on the activated alkene is usually a ketone making it an enone but it can also be a nitro group.
As originally defined by Arthur Michael,[3][4] the reaction is the addition of an enolate of a ketone or aldehyde to an α,β-unsaturated carbonyl compound at the β carbon. A newer definition, proposed by Kohler,[5] is the 1,4-addition of a doubly stabilized carbon nucleophile to an α,β-unsaturated carbonyl compound. Some examples of nucleophiles include beta-ketoesters, malonates, and beta-cyanoesters. The resulting product contains a highly useful 1,5-dioxygenated pattern.
Classical examples of the Michael reaction are the reaction between diethyl malonate (Michael acceptor) and diethyl fumarate, that of mesityl oxide and diethyl malonate, that of diethyl malonate and methyl crotonate, that of 2-nitropropane and methyl acrylate, that of ethyl phenylcyanoacetate and acrylonitrile and that of nitropropane and methyl vinyl ketone.
The Michael addition is an important atom-economical method for diastereoselective and enantioselective C-C bond formation. A classical tandem sequence of Michael and aldol additions is the Robinson annulation.
Hydride Reductions of Aldehydes and Ketones 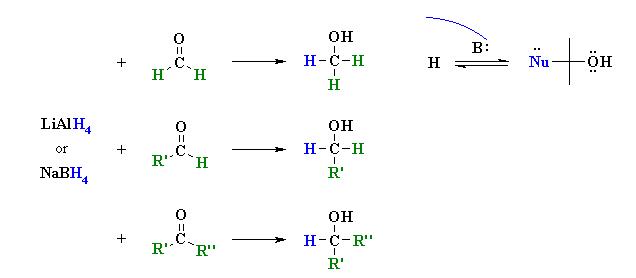 [
[
Reactions usually in Et2O or THF followed by H3O+ work-ups
Reaction type: Nucleophilic Addition
Summary
Aldehydes and ketones are most readily reduced with hydride reagents.
The reducing agents LiAlH4 and NaBH4 act as a source of 4H- (hydride ion).
Overall 2 H atoms are added across the C=O to give H-C-O-H.
Hydride reacts with the carbonyl group, C=O, in aldehydes or ketones to give alcohols.
The substituents on the carbonyl dictate the nature of the product alcohol.
Reduction of methanal (formaldehyde) gives methanol.
Reduction of other aldehydes gives primary alcohols.
Reduction of ketones gives secondary alcohols.
The acidic work-up converts an intermediate metal alkoxide salt into the desired alcohol via a simple acid base reaction.
Nucleophilic Addition Reactions
Strong nucleophiles (anionic) add directly to the C=O to form the intermediate alkoxide. The alkoxides are then protonated on work-up with dilute acid.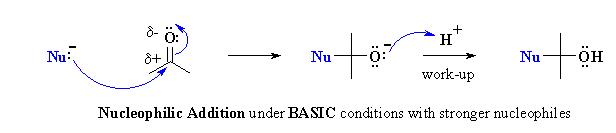
Examples of such nucleophilic systems are : RMgX, RLi, RCºCM, LiAlH4, NaBH4
Weaker nucleophiles (neutral) require that the C=O be activated prior to attack of the Nu.
This can be done using a acid catalyst which protonates on the Lewis basic O and makes the system more electrophilic.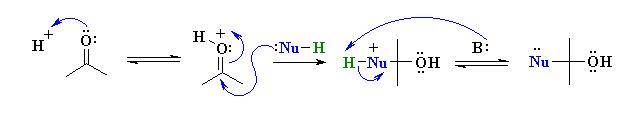
Examples of such nucleophilic systems are : H2O, ROH, R-NH2