The following is a graph plotted between the vapor pressure of two volatile liquids against their respective mole fractions
 Which of the following combinations are correct?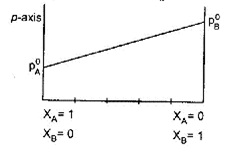
(1) When {{x}_{A}}=1~and~{{x}_{B}}=0,~then~p=p_{A}^{0}
(2) When {{x}_{B}}=1~and~{{x}_{A}}=0~then~p>p_{A}^{0}
(3) When {{x}_{A}}=1~and~{{x}_{B}}=0~then~p<p_{B}^{0}
(4) When {{x}_{B}}=1~and~{{x}_{A}}=0~then~p=p_{B}^{0}
Which of them is incorrect? a) 1 b) 2 c) 3 d) All are correct statements
-
UP 0 DOWN 0 0 0
