this is something really important and good.. THanks a lot ...
Hey frnds,
I have collected some important and not-so-known points of organic chemistry from my notes and assembled them here.
GOC
1-Carbocation stability- Ring expansion should always be given higher priority than tertiary carbocation.
2- If some unknown reactants are given to U and if U cant decide how to start, always give first priority to an acid-base reaction.
3- Carbene(:CCl2)- Triplet is more stable than singlet.
For :CF2- Singlet is more stable than triplet as it can have back-bonding in singlet carbene due to the presence of an empty orbital.
4- Benzyne - The inductive effect(and not resonance) of the group attached to benzene decides the orientation of incoming reagent becoz the triple bond of benzyne is sigma bond and not pi-bond.
5- In Ph-NH3+, NH3+ is slightly more para directing than meta(EXCEPTION).
ISOMERISM
1-Tautomerism - Acidic H should be released only from saturated C and not from unsaturated C.
2-Geometrical isomerism is not applicable in alkynes.
3- For a compound to be optically active, it should lag both plane of symmentry as well as centre of symmetry.If anyone of them is present in compound, it wud become optically inactive.
ALCOHOL,PHENOL & ETHER
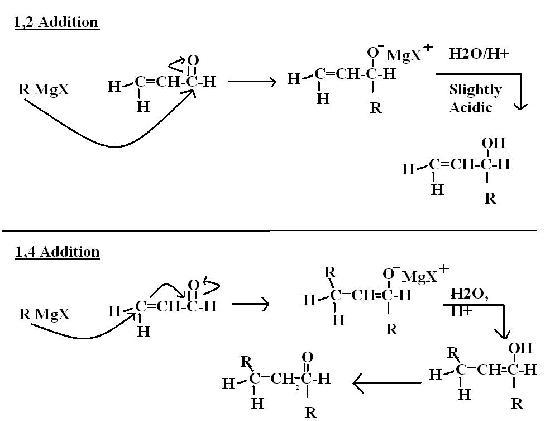
1- 1,2 v/s 1,4 addition
Mechanisms are shown in figure.
1,4 product - Thermodynamically stable and is produced in long duration(for example-in reaction with NaBH4).
1,2 product- kinetically stable and is produced in short duration(for example- in reaction with LiAlH4).
=> Generally thermodynamically stable product is given.
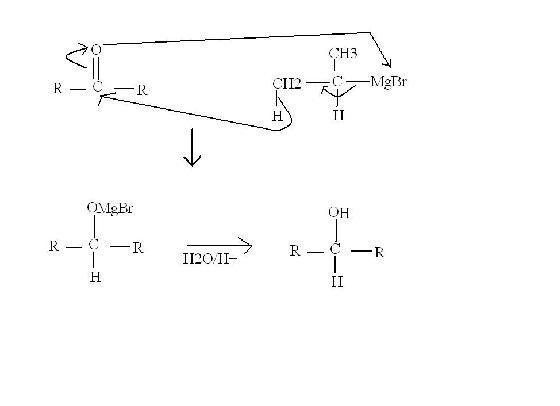
2- U all must have read the preperation of alcohol by ketone and grignard reagent. There's an exception to this reaction-
Ketone = Me2CH - CO - CHMe2
Grignard Reagent = Me2CH-MgBr
In the reaction with these reagents, alcohol is not formed by the usual mechanism in which R- attacks C=O. In this reaction, the ketone is reduced by H- from grignard reagent. The concerted mechanism in shown in figure.
If we take a ketone/G.R. bigger than this one => No reaction.
If we take a ketone/G.R. smaller than this one => Normal reaction
Means, the steric hindrance of both the molecules matter and not only anyone.
3 - Luca's test- We all know that 3o alcohols give immediate turbidity with luca's reagent. BUT there are 2 more alcohols which are not 3o but still give immediate turbidity with Luca's reagent(dont ask me the mechanism ). They are-
Ph-CH2-OH and CH2=CH-CH2OH
4- Williamson's Etherification - The halide used should not be aryl halide becoz the reaction wont take place due to partial double bond character of C-X bond by resonance.
Till now I have been able to collect only these points. Will post more as soon as I assemble them.
Hope these points help U in ur exams.
All the best!!!
-
UP 0 DOWN 0 1 4

4 Answers
Superb, Prateek. Really useful. The only thing that i knew already was the 4th one.
Nice work.........keep 'em coming [4]