carl is right,no chance of trans there.
THE HIDDEN THINGS ARE FOR MY REFERENCE TO CONFIRM ANSWERS THEY R IN NO WAY RELATED TO ANS OR QS
(!) Mn 2+ can be oxidised to MnO4- by --- --> 13Fbl 1
(A) SnO2
(B) PbO2
(C) BaO2
(2) The reaction which proceeds in the forward direction is 13Ob 4
(A) Fe2O3 + 6Hcl = 2Fecl3 + 3H2O
(B) NH3 + H2O + Nacl = NH4cl + NaOH
(C) Sncl 4 + Hg2cl2 = Sncl2 + 2Hgcl2
(D) 2CuI + I2 + 4H+ = 2Cu2+ + 4KI
(3) Among Ni(CO)4 , [Ni(CN)4]- and Nicl42- 13 Obj 5
(C) Ni(CO)4 and [Ni(CN)4]2- are diamag and Nicl42- is paramag
(B) Nicl42- and [Ni(CN)4]2- are diamag and Ni(CO)4 is paramag
(4) In the dichromate dianion 13obj 13
(A) 4 Cr --O bonds are equivalent
(B) 6 Cr --O bonds are equivalent
(C) All Cr--O bonds r equivalent
(D) All Cr --O bondas are non equivalent
(5) Photobromination of 2-methyl propane gives a mixture of 1-bromo - 2 - methyl propane and 2-bromo - 2 - methyl propane in the ratio 9:1
17T/F2
TRUE / FALSE
(6)Hydrogenation of the adjoining compound in the presence of poisoned palladium catalyst gives :
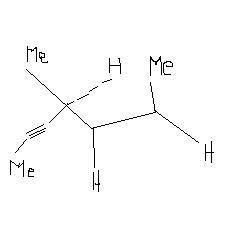 17Obj17
17Obj17
(B) An optically inactive compound
(C) A diastereomeric mixture
(7) 2-hexyne gives trans - 2- hexene on treatment with : 17obj 24
(A) Li/NH3
(B) Pd/BaSO4
(C) LiAlH4
(D) Pt/H2
-
UP 0 DOWN 0 0 20

20 Answers
k thanks Carl
The explanation given was
" " due to absence of
hydrolysis of Fecl3 backward rcn doesnt take place " "
But I dint understand this explanation
How can v say that hydrolysis doesn't take place ??
@Carl & lubu thanks [159]
Got my mistake and ya agree with the given ans
sO FINAL ANS IS ONLY OPTICALLY INACTIVE
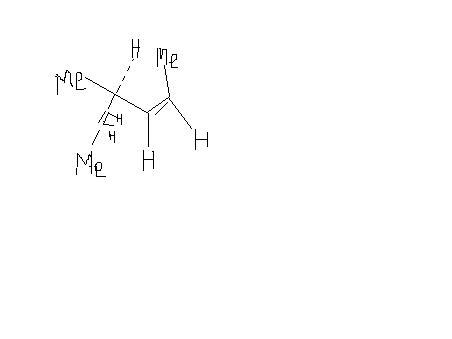
Sorry, i had made d structure of Alkene but wrote that alkane. I am sorry for dis mistake,But d structure says everything.
@lubu : R u sure of it ???
Coz in the Arihant past year's book the ans is given out to be some alkene it self
alkene --> optically inactive as well as diastereomers
coz it has cis trans compds which r diastereomers
I think in Qno.6 partial hydrogenation will take place.So from -C≡C- alkane is formed.
there fore a optically inactive compund will form.
Ques 4..modified..suppose we have anion like this [HCr2O7] -
Now comment on the bond lengths..
ya thanks Pritish and utd4ever for the 7th qs
and Carl and Govind for the remaining qs
Can anyone proceed with 6th qs also plzz!!!!
7 th question the reaction is birch reduction in which lithium ionizes giving an electron which attaches to the triple bond and so we have an intermediate in which there is an anion and radical and for max stability of theis intermediate these two must be far away and so opp sides and so hydrogen attach from opp sides giving you a trans compound ...
ans for the first question is b becoz as we go down the group inert pair effect increases so Pb has more tendency to exist in +2 rather than Sn ....
@Govind : k agreed with 3 and 5 also (Though given answers r contradicting)
If any one agree with the given answers plzz post ur explanations
3 --> B
5 --> True
Ans 2..A can be the answer..basic oxide + Acid...
Ans 5 False...bromine radical is very selective so tertiay halide will be the major product
@Carl : Even i got the same ans as urs
But ans given is C
I'm referring Arihant Past year