2) is it 0.2199??wild guess + too much aapx....so tll whether right?
Q1) Using Vander waal's equation of state, find the pressure at which the PV vs P curve acquires minima for 1.0 mole of oxygen gas at 0°C . a = 1.36 L2 atm mol-2 and b = 32 cm3mol-1
Q2) A beam of X-rays is scattered by loosely bound electrons at 45° to the direction of beam. The wavelength of scattered beam is 0.22 A°. What is the wavelength of incident beam ?
-
UP 0 DOWN 0 0 9

9 Answers
Using,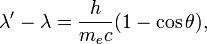
λ is the initial wavelength,
λ′ is the wavelength after scattering,
c is the speed of light, and
θ is the scattering angle.
here angle is 45 degree,,,,and λ′ is 0.22 as given by q,...........and h/mc is equal to 2.43×10−12 m.so u can find λ
WIERD CALCULATION....CALCULATOR SE BHI KARNE MEIN I WAS CONFUSED! [3][3]
that was gud but is the formula available in any theory buk or so...
where did u find that formula ?
Yeah that formula is there in many books. Infact its there in BITSAT arihant book :P
So this means this question was Manmay's doubt as he didn't know the formula..:P
bhaiya please give some hint regarding q 1.
i got ans expression of PV using vanderwaals formula but am getting stuck when trying to differentiate w.r.t P