49
49"Why........?" is the question of the day! [3]
 11
11a is correct because n-factor can be fractional!! You can lose 0.5 moles of electrons but not half of an electron..
Tui sei ICP r concepts gulo kei ghantachis??
 11
11Sei Dilshad sir er matrix match ta.. :-)
 49
49I used to copy down all important questions that I liked in a copy.. this is from there.. maybe from ICP classes :)
 1
1If 1 mole of reactant loses 2 moles of electrons
This implies that - 1xNA molecules of it loses 2xNA electrons[NA is the Avogadro No.]
Hence........1 molecule loses 2 electrons.
in Both of these cases n-factor is the same....so the answer seems to be (c)..
but i`m nt 100prcnt sure about my answer,i think i`m missing something....please clarify??
 49
49Answer these urself:
can n-factor be a fraction?
 49
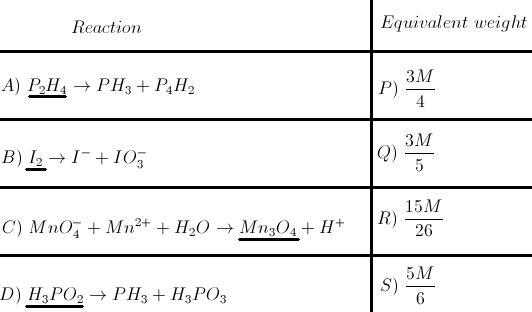
49ANSWER TO SECOND QUESTION:
A-S; B-Q; C-R; D-P