?????
1..IN WHICH OF THE FOLLOWING BONDS re not equal
1.SF4 2.SiF4
3.XeF4 4..BF4
GIVE EXPLANATION [2]
-
UP 0 DOWN 0 0 5

5 Answers
Apply VSEPR to find their shapes...except SF4, they are square planar(which means equal bond lengths). Due to lone pair in S, the bond angle decreases.
SF4 -- see-saw shaped compound sp3d hybridisation so it has two kinds of S-F bonds axial and equatorial
XeF4 Square planar sp2d
SiF4 sp3 it's tetrahedral and not square planar
and BF4- also tetrahedral sp3
In square planar compounds hybridisation is sp2d so they involve participation of d-orbitals whereas in tetrahedral compounds hybridisation is sp3 and they do not have participation of d-orbitals..
square planar compounds hybridisation is not sp2d govind its dsp2 na??
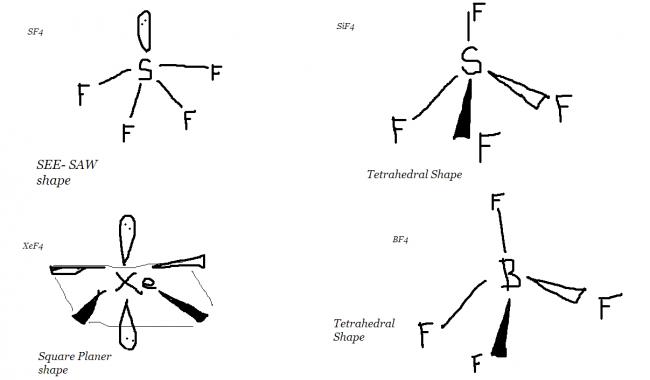
SF4--->sp3d
SiF4---->sp3
Acc to VSEPR
(6+4)/2=5 and 1 lone pair => see saw shape....here the see saw shape is a bit hindered due to presence of lone pair electrons
XeF4----> sp3d2
acc to VSEPR
(8+4)/2=6 and 2 lone pairs => square planar
BF4- -----> sp3
Acc to VSEPR
(3+4+1)/2=4 and 0 lone pairs => tetrahedral shape
Each angle of tetrahedral = 109.5°
Each Angle of square planar= 90°
Angles of see saw shape = http://en.wikipedia.org/wiki/Sulfur_tetrafluoride