neone?
Can someone help me why the following compounds do not show tautomerism.
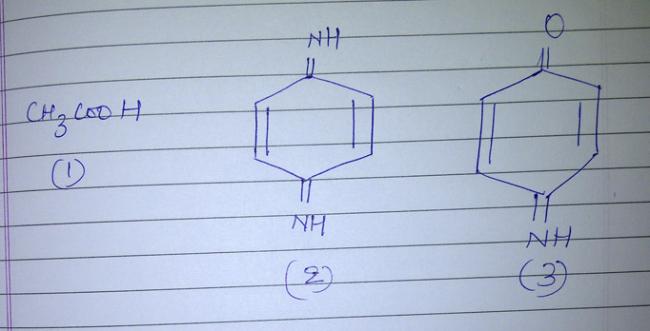
-
UP 0 DOWN 0 0 5

5 Answers
In CH3COOH, due to equivalent resonance between the two oxygen atoms, they will not take part in resonance with any other charged species.. Hence, the negative charge that comes on the carbon atom doesnt shift from carbon and hence, resonance cant take place. Hence no tautomerism.
In case of the other two compounds, all the carbons in the ring are sp2 hybridised. Now when hydrogen is taken away by the base, the negative charge is contained in an orbital that is in the plane of paper. It also cannot take part in resonance and charge cannot move to a more stable position via resonance and hence, again tautomerism will not occur!
certainly there will be a tautomer for acetic acid , but dont u think it will be the same compound??
:P
if there are three double back to back in a ring then the geometry wont be satisfied cause the gemetry of alternat e double bond is sp2 which is co-plannar , and if three double bond come together it will become sp which is geometrically not possible. hope u understand it.....