 1
11. It must be NH3 >PH3>AsH3>SbH3
 1
12.ethyne has strongest sigma bond i think bcoz it sp hybridised .......since the s charcter is 50% its bond is more strong
 1
11. NH3 >PH3>AsH3>SbH3 as polarity is inversely prop..to size of cation.. 2. ethane...sp3 ....high p character
 1
1q.no 2:
ethyne has the strongset sigma bond
Reason:
the hybridization of C atoms in ethyne is sp
more the s character more symmetrical the atom as well as more compact
hence due to more compact atom the bond length is the least in case of ethyne and hence the bond is strongest
 1
1well,i thought so too but the ans given is(arihant)-
1.NH3>AsH3>SbH3>PH3.
2.ethane-due to greater overlapping of sp3-sp3 hyb orbitals.
R they right??
 1
1avinav we are not talking about dissociation energy......we are just talking about the STRENGTH OF SIGMA BOND and THERE IS JUST ONE SIGMA BOND BETWEEN THE 2 C ATOMS in ETHYNE HENCE YOUR ANSWER IS CORRECT BUT REASONING WRONG! plz read the question carefuly
 106
106Q2. ans is ethane.. ishan we are talking about only the sigma bond.. excluding the pi bonds (read the question) sigma bond is strongest for sp3 orbitals as they are the most directional (most p-character) hence the overlap is max. So the sigma bond in ethane is strongest
But the C-C bond in ethyne is strongest because of the addition of two extra pi bonds
 1
1ok thanx..i got some logic for the order of polarity-in NH3 nitrogen is more electronegative than hydrogen bt in PH3 and the rest,P is less electro-ve than H so the moment due to lp n that due to bond is oppositely directed leading to reduction in polarity.but hw 2 arrive at the exact order??
 1
1Both are able to pick up protons, but NH3 is a more polar molecule due to the dipoles between the hydrogen and the nitrogen (electronegativity difference of 0.9). Picking up a proton gives the molecule a charge, but removes the dipoles. PH3 is non-polar, so it doesn't require this.
 1
1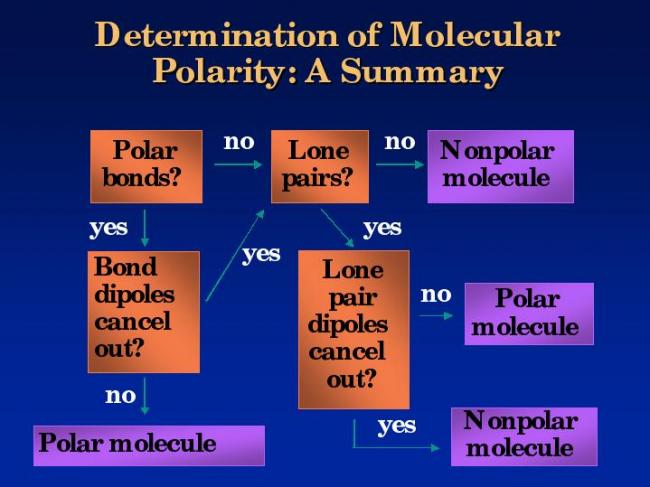
This might help you to understand the conditions for polarity
 24
24what a concept map dude....[1]
