the gas A is CO2 ..CO2 on reacting with coke forms CO
so gas B is CO..
now in the carbonyl complexes the metal remains generally in the grond state...
2.6 moles of KMnO4 will absorb 13 electrons in acidic medium..
So Fe→Fe3+ three electrons released
CO → CO2 2 electrons released..
so the formula of the complex is[{Fe(CO)5]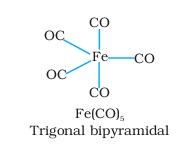
with 8 d electrons and it is dimagnetic..hybridisation dsp3
by o-phen do u mean o-phenanthroline??
well i think o-phenanthroline is a bidentate ligand..so most probably it will ccupy equatorial position..so total number of geometrical arrangements shud be 1..
In manufacture of iron a gas A is formed in zone of combustion of balst furnace.Gas A reacted with coke in zone of fusion to form another gas B.x moles of B react with iron at 200 C and 100 atm pressure to form C,one mole of which requires 2.6 moles of KMnO4 in acidic medium to oxidise into Fe3+ and CO2
1> If 2 moles of B present in complex is substituted by o-phen ,the total number of possible geometrical arrangements in complex are ?
2> The d orbitals involved in formation of complex are ?
-
UP 0 DOWN 0 0 2

2 Answers
govind
·2010-02-08 00:35:48