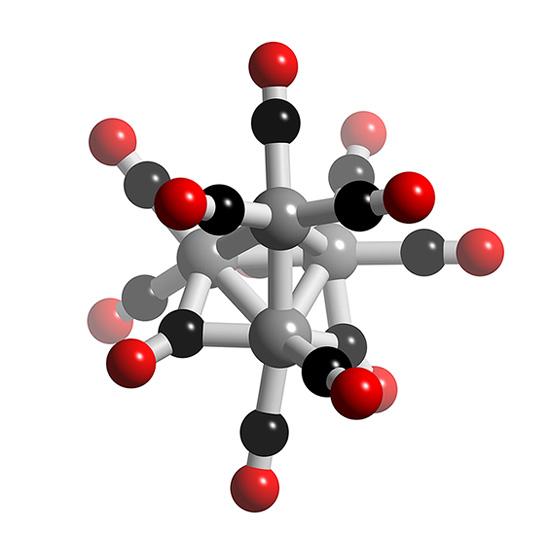
In the figure grey represents Co atom, Black carbon atom and red oxygen atoms...
so 6 metal-metal bonds are there...
1.calculate the no. of metal-metal bond of Co4(CO)12 if complex follows 18e- rule ????
-
UP 0 DOWN 0 0 4

4 Answers
How can this prob be solved in IIT point of view
I mean other than just remembering??
I dont think there is any other method to do this question except hit and trial...if this question comes in integer type format then it will be very difficult to solve..it it comes in an single correct objective type then u can use the options to get an idea of the structure...Actually in my attempt i reached till four metal - metal bonds..and that sharing of carbonyl grp b/w two metals..but that was not satisfying the EAN rule..so i searched on the net to look for the structure..coz i didnt expect more than four Metal-metal bonds ..coz that will be very sterically hindered structure but was surprised to see that was the answer...
@rahul
The 18 electron rule (also known as the Effective Atomic Number Rule or EAN rule) was originally proposed by N.V. Sigwick when extending the octet rule proposed by G.N. Lewis in applying it to organometallic compounds. The idea behind both of these rules is that in a compound the sum total of all of the electrons would have the configuration of a noble gas.
Formula for calculating EAN = atomic number of metal - charge on the metal(here the charge is taken with sign) + number of ligands around metal * 2
the complexes satisfying the EAN rule are said to be more stable...but there are many exceptions of this rule..