 106
1061. If Boron had d-orbitals then it would have been possible to form compounds with +5 oxdn state. But as it doesnt have, B can show +3 oxdn state only. When B accepts a pair of electrons then it can show max. covalence of 4 as in [BF4]+
 1
1ya..2) is also correct
the correct explanation would be its structure i suppose
its given in JD LEE..i am sure u || love its structure, atleast i do :)
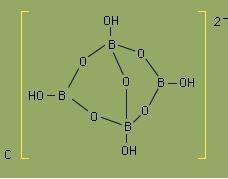
See more questions can be made from Borax molecule
like hyb of B atoms in Borax can be asked, it is both sp2 and sp3 ..see urself
and ya forgot to mention, two cations are [Na(H2O)4]+
 1
11)Boron has an EC of 1s2 2s2 2p1 putting it in and making an Electron unpaired
we see that it has an
O.State of +4 in presence of d-orbital it would have been Five
2)Borax here is sp2 and sp3 hybridisaiton and the diagram is as given above
