ans should be the last option
just getting a faint idea that it may be the third one !
Aqueous silver nitrate is treated with successive additions of:
(i) an excess of aqueous potassium chloride;
(ii) an excess of aqueous ammonia;
(iii) an excess of aqueous potassium bromide.
Which of the following diagrams represents the mass of precipitate produced after each addition?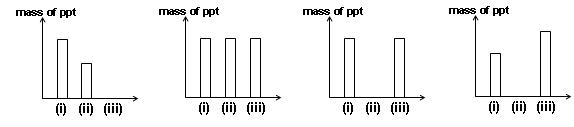
-
UP 0 DOWN 0 1 6

6 Answers
look , (ii) is soluble,,,the diamine compound ! hence we have to choose between the third and fourth option !!!! with some hit and trial i preferred to go for the fourth one ! but i cannot figure out the exact nature of the ppt in (iii)......can you please help me out mr. mentor !
As far as i remember, the solubility product of AgCl > Ksp of AgBr ....(can't see ny other way of comparing right now)
So, yes most probably the last one is correct...
yes,
solubility of AgX decreases down the group,
So AgBr is less soluble than AgCl hence ppt of AgBr is more than ppt of AgCl
and the complex Ag[(NH3)2]+ is water soluble so no ppt
hence (d) is correct