DIPOLE MOMENT IN AROMATIC SYSTEM
Let us consider some examples
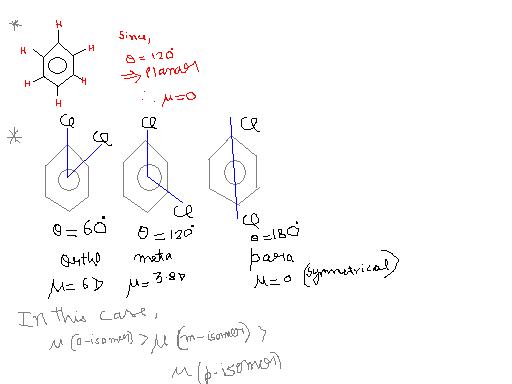
Dipole moment in aromatic system
can any 1 could explain?
THANKS in advance
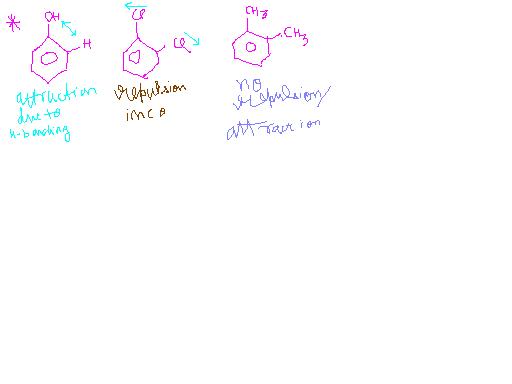
This is due to dipole -dipole repulsion in ortho isomer that inc bond angle geater than 60° and thus dipole moment dec.
theta dec, dipole moment inc
Dipole moment dec, no change in actual value.
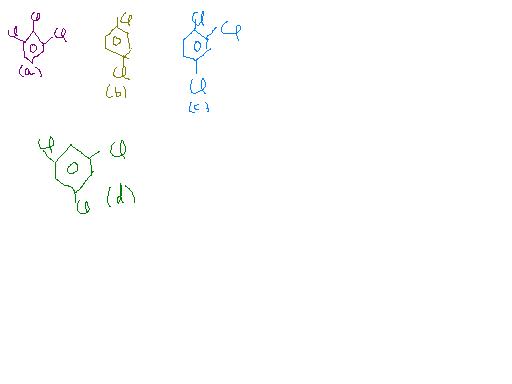
(b) and (d) are symmetrical and thus resultant dipole moment is zero.
In (a) bond moments are towards the same direction but in (c) there is a net dipole at the c2 position. Thus the dipole moment of (a) is max.
(b) = (d) < (c) < (a)
tushar:
with reference to #2, the dichloro substituent has higher dipole moment not the dimethyl substituent one