ans is b
can u plz elaborate ur explanation with a suitable diagram...
why is it not 2,4,6
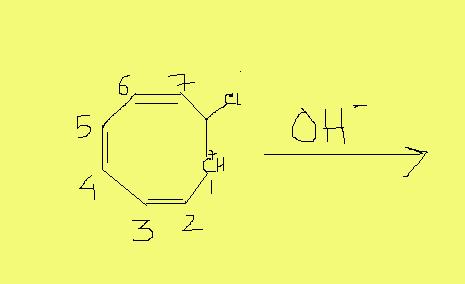
predict the site of reactivity for the above reaction
a) 1,2,3,4,5,6,7
b)1,3,5,7
c)2,4,6
d)1,4,7
ans
please explain.....
-
UP 0 DOWN 0 0 6

6 Answers
abhimanyubhardwaj
·2010-01-15 03:06:25
i think its d,as oh is a nucleophile and therefore electron rich,it wud search the site wich easily accepts electron,nd 1,4,7 might b the right site,if not then may b its due to +i effect
SANDIPAN CHAKRABORTY
·2010-01-15 05:29:42
Asish Mahapatra
·2010-01-20 01:31:44
simple, draw the resonance structures...
see where the positive charge CAN appear. ull get 1357 only
swaraj jena
·2010-01-20 10:33:09
will not the hydride shift occur from the carbon containing Cl to give an 3° carbcation with more stability and resonce stabilized by Cl
