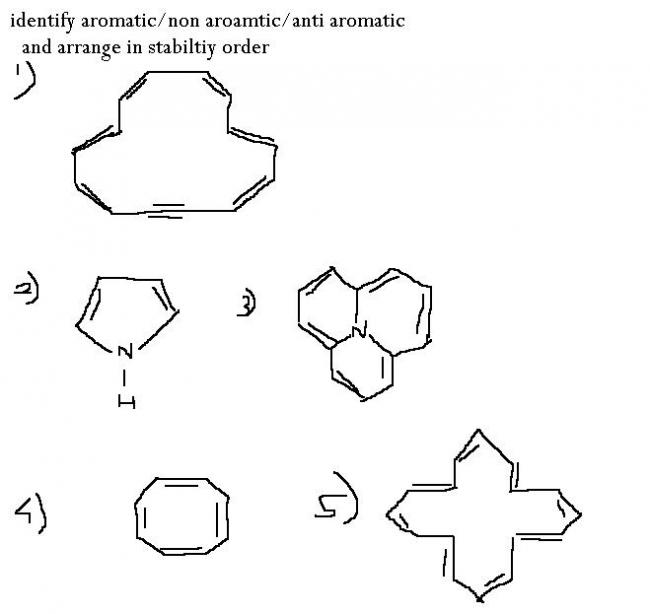
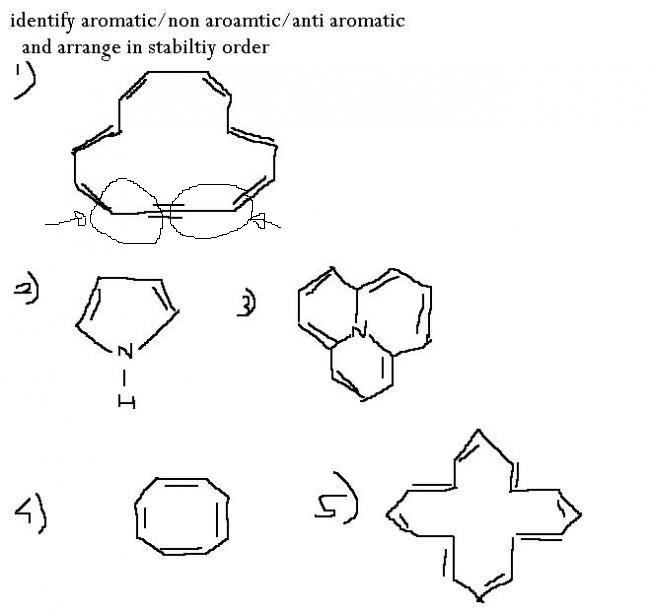
1)
2)Non aromatic
3)Non aromatic
4)Anti aromatic
5)
13 Answers
first of all i not sure abt antiaromatic srry for tht::::
1)how is structure possible wid C making 5 bonds..
2)aromatic(as all C are sp2 hybridised nd it follows huckle rule also).(nitrogen has lonepair).
3)aromatic(as all C are sp2 hybridised nd it follows huckle rule also).(nitrogen has lonepair).
4)antiaromatic.
5)antiaromatic.
Of course 2) is aromatic...nitrogen participates in resonance, making the structure planar. Though I'm not sure about 3), nitrogen being in that area, its lone pair might not be available to all 3 rings? (thus hindering aromaticity)
no dude....this is standard way of drawing...
u can understand like this..
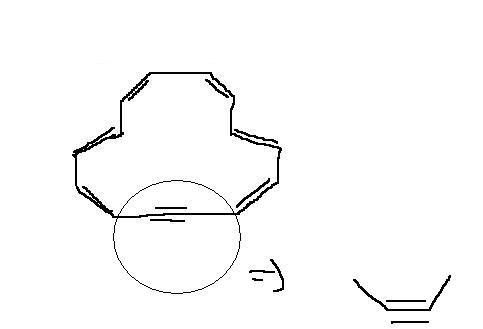
srry bhaiiya but i was wrong for the 4th one cozz its an aaception it is non aromatic compound refer this link::::::http://targetiit.com/iit-jee-forum/posts/what-s-antiaromatic-14884.html
its structure is non planar as given below
srry for the mistake
[7][7][7][7][7][7][7][7]By the way can anyone tell me why this structure is non planar ???????????????[7][7][7][7][7][7][7][7]
okies.
methinks::
1.a hydrocarbon
2.aromatic
3.aromatic
4.a hydrocarbon
5.a hydrocarbon