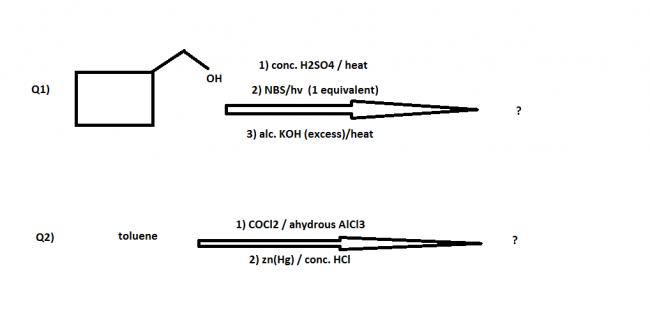
In Q1)
STEP 1: OH- would cleave due to H2SO4 leaving a positive charge on carbon. Then there would be RING expansion forming CYCLOHEXENE
STEP 2: With NBS at 2 carbons next to double bonded carbon Br- would substitute at place of hydrogen.
STEP 3: With alc KOH which is a dehalogenating agent form BENZENE