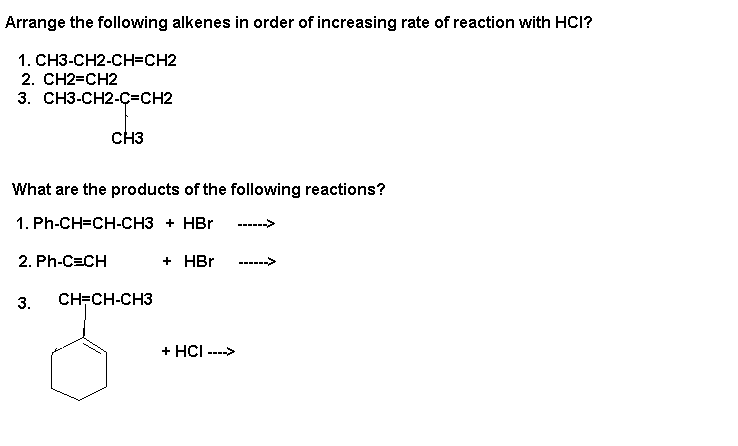
Rate of reaction-3>1>2
See the reaction will proceed with the formation of carbocation.......So SN1.....ab SN1 mein 3°>2°>1°...I hope this is d answer.....
11 Answers
Anurag Ghosh
·2013-08-05 05:07:36
Anik Chatterjee
·2013-08-05 05:49:39
1.Ph-CHBr-CH2CH3
2.Ph-CBr=CH2
3.??in the propene branch,the ring is bonded to 1st or 2nd carbon???
 Dwijaraj Paul Chowdhury obviously 1st carbonUpvote·0· Reply ·2013-08-05 06:35:37
Dwijaraj Paul Chowdhury obviously 1st carbonUpvote·0· Reply ·2013-08-05 06:35:37
Swarna Kamal Dhyawala
·2013-08-05 07:33:46
3) 1-chloro,1-propenyl ,cyclo hexane
 Anurag Ghosh An idiotic question but is Ph a source of electrons???....getting confused??
Anurag Ghosh An idiotic question but is Ph a source of electrons???....getting confused?? Anurag Ghosh And Swarna wat did u do in d third one???.....give me d mechanism.....
Anurag Ghosh And Swarna wat did u do in d third one???.....give me d mechanism.....
Harsh Bharvada
·2013-08-05 19:36:25
Swarna ur answer is wrong....try again..
 Swarna Kamal Dhyawala then what is the answer
Swarna Kamal Dhyawala then what is the answer

Swarna Kamal Dhyawala
·2013-08-06 06:34:46
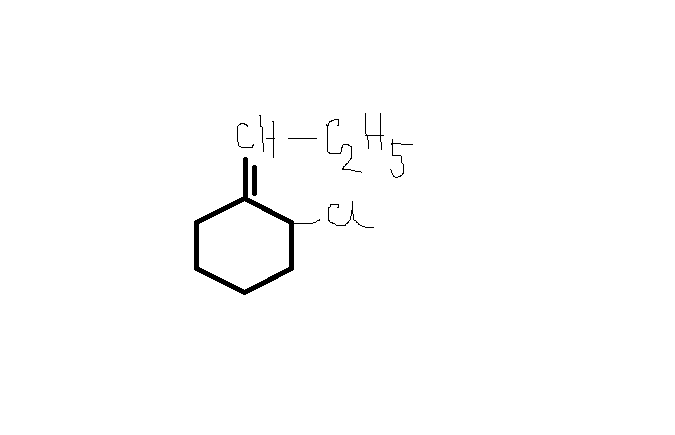
 Jeet Sen Sharma this cannot be correct atleast i think so.....
Jeet Sen Sharma this cannot be correct atleast i think so..... Prashant Kulthia this is the correct ans accrding to me because the carbocation formed is very stable...
Prashant Kulthia this is the correct ans accrding to me because the carbocation formed is very stable...

Divyans Jhunjhunwala
·2013-08-07 13:08:03
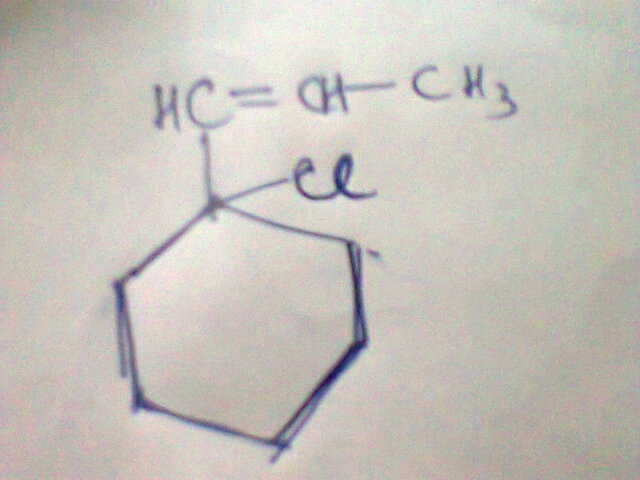
maybe ans wud be 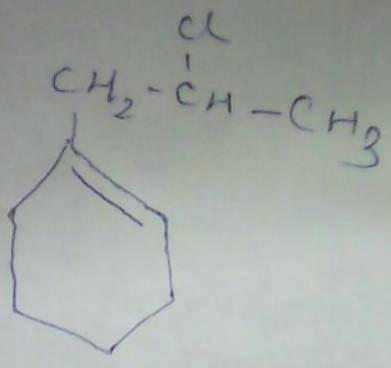
 Divyans Jhunjhunwala can this be the reason...
as before reaction the ring having a π(pi) bond follows (4n+2)π rule so its aromatic but if we remove the bond we are disturbing the aromaticity so i think this wud be the correct answer.
Divyans Jhunjhunwala can this be the reason...
as before reaction the ring having a π(pi) bond follows (4n+2)π rule so its aromatic but if we remove the bond we are disturbing the aromaticity so i think this wud be the correct answer.
Anurag Ghosh
·2013-08-07 18:14:45
Hmmmm...Divyansh lagta hai tu r8 hai......didn't observed d pi bond inside...:P We r disturbing d aromaticity of d compound......
 Divyans Jhunjhunwala The decision Rests with harsh about the correct answer... :-)
Divyans Jhunjhunwala The decision Rests with harsh about the correct answer... :-) Swarna Kamal Dhyawala @anurag from were can u see it is aromatic
Swarna Kamal Dhyawala @anurag from were can u see it is aromatic
Harsh Bharvada
·2013-08-08 00:14:36
i just cant believe dat none of got it right......hehehe...cant stop laughing!!!!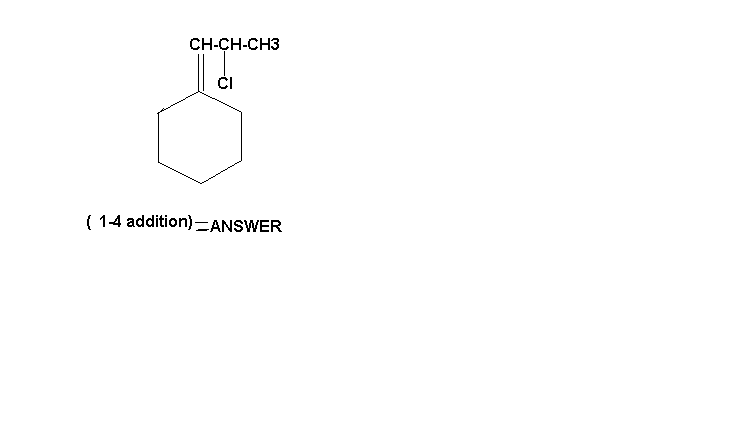
 Swarna Kamal Dhyawala in this case we r getting 2 degree carbocation but in 1-2 addition we get three degree carbocation
Swarna Kamal Dhyawala in this case we r getting 2 degree carbocation but in 1-2 addition we get three degree carbocation  Jeet Sen Sharma are u sure....?? this is the ans..?? y ??
Jeet Sen Sharma are u sure....?? this is the ans..?? y ??
Manish Shankar
·2013-08-08 01:02:21
It is not aromatic all are not sp2 hybridized
Answer given by swarna seems correct to me