bcz H attached to N was more acidic , than that to C ,and it forms more stable 5 membered rings,
frndz say the reason why the taoutomer for the following is the 2nd one??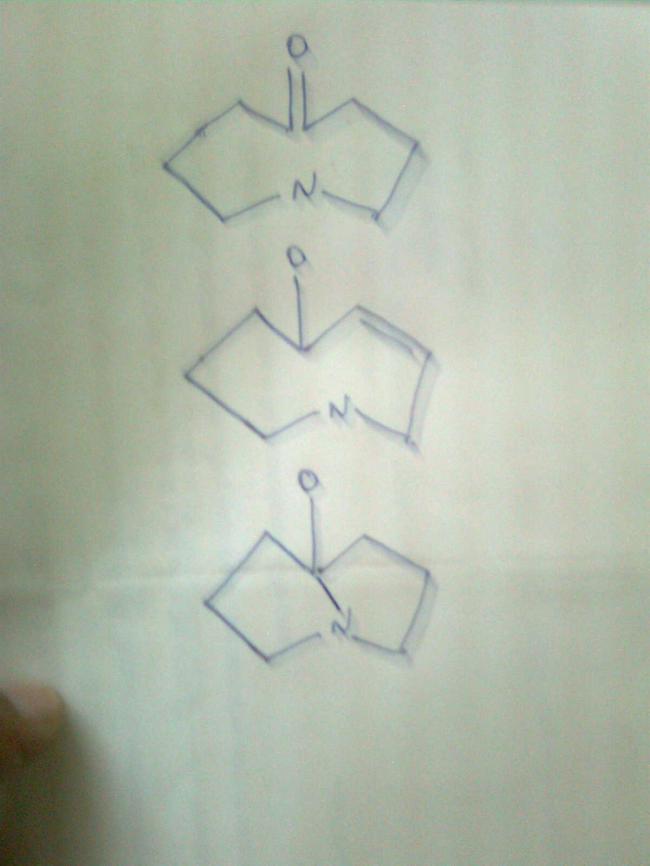
but generally it shld be taken from alpha carbon but this differrent but y??
-
UP 0 DOWN 0 0 8

8 Answers
What is the 3rd figure about?
The valency of oxygen is not satisfied in 2nd and 3rd figure
sry itz Oxygen connected to H and itz keto enol tautomerism i missed out the H connected to O so now you tel the reason??
i can say it bcz i studied it somewhere , and H attached to O > N>C (wen all sp3) , acidity wise
BUt in dat tautomersimm the hydrogen is alwayz taken from alpha hydrogen na???but in dis case y N hydrogen is attached??as u said itz more acidic k but is it an experimental fact or thrz any other reason behind it????
how will oxygen come to kno wich H is of Carbon and wich H is of Nitrogen ? it will just abstract the one wich is more acidic ...
btw here i think mechanism is such that first N attacks like a nucleophile on the carbonyl carbon , and then oxygen abstracts H .
dont think of tautomerism like in #8. by definition tautomers are functional isomers in equilibrium, and they may interchange thru many ways , not necessarily abstraction of H frm alpha carbon .... wat u r thinking is just keto enol tautomersim , but thats not the only type possible ...