Bond Length is inversely proportional to bond order
Calculate their bond orders
Q. Correct order of bond length is
(a) CO>CO+ (b) NO+ > NO (c) N2>N2+ (d) O2+>O2
ans: (a)
Bond Length is inversely proportional to bond order
Calculate their bond orders
yeah can u tell me the bond order of 1st one? like the structure of CO and CO+ ?
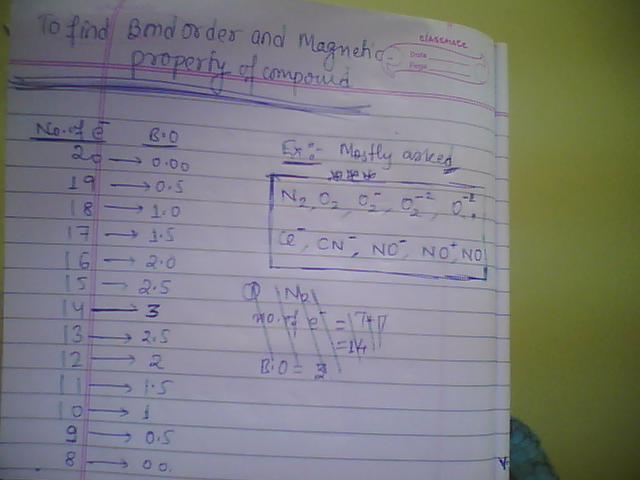
This is the very easiest method to find Bond order bond length and bond strength.
Now in question
CO+ = 6 + 8 - 1 = 13 electrons...so BO = 2.5
CO = 14 electrons so BO = 3.
Now If BO is high then strength will be high and Bond length will be low...
So First one incorrect....
For other options calculate the respective no. of electron considering the outer most electrons...match with column given in image and get ur answer...
With regard
YAgyadutt
n1=sum of valence electron of all the atoms of the species +- net charge on species
n2=(8xno of atoms other than H) +(2x no of H atoms)
then bond order is given by=(n2 -n1)2
CO
n1=4+6=10
n2=2x8=16
BO OF CO=(16-10)/2 =3
CO+
n1=4+6+1=11
n2=2x8=16
BO OF CO+=(16-11)/2 =2.5
BO of C0 >BO of CO+
HENCE bond length of CO+> bond length of CO .........similarly do it for others
i felt only one thing the cations are smaller in size than its parent atom,so i think a and c shud be rite.neone correct me if i am rong.
....what's problem man...both the post are clearly written...then also u are not getting... i am clearifying once again...
Step 1: You have to find out the toatl no of electrons..
ex. CO+
C has 6 e- O has 8 6- and there is + charge so one electron is removed
hence total no. of electron is equal to 8 + 6 - 1 = 13.
In my table what is its bond order...it is 2.5
For CO what will be? it will be 3 coz electron are 14 clear???
So Bondlength is inversely proportinal to Bondorder
Hence CO < CO+
secon ex. NO+ and NO
NO+ = 7 + 8 - 1 = 14 hence B.O = 3
NO = 8 + 7 = 15 hence B.O = 2.5
Again NO>NO+ in bond length.
ex:3] N2+ and N2
N2 = 14 B.O = 3
N2+ = 13 B.O = 2.5 hence N2+>N2 in case of Bond length..
ex:4] O2+ and O2
O2 = 2x8 = 16 B.O = 2
O2+ = 15 B.O = 2.5
hence O2 > O2+ in Bond length...
Cheers body i have solve all the option...
thats what I and maish sir are saying dude..answer is none of these.....
Ohhhoooooooo
Manish sir has written that i m getting none of these...
I understand this as...mujhe dono me se koi solution samajh nahi aa raha hai..english ki to maine....
BO of CO+ is 3.5 indeed... (given in JD LEE) It is not explained by MO theory
http://www.chemicalforums.com/index.php?topic=37957.msg145632#msg145632
wow....yagyadutt has given us a fantastic shortcut...really time saving !!!!! great job dude !