u hav to see the degree of freedom for those molecules and calculate...
at higher temperatures u have to take into account the vibrational K.E. as well.
What are the Cv values for
CH4 and CO2@ 400K,1200K and 1400k
please tell the method of calculation
u hav to see the degree of freedom for those molecules and calculate...
at higher temperatures u have to take into account the vibrational K.E. as well.
for CO2 and H20 deg of freedom is 6 at ordinary temp.
at high temp, it is 7 (for vibration)
at ordinary temp,
Cv for both = 4R
at high temps(1200K and 1400K), it is 5R.
isn't deg of freedom for co2 at ordinary temp 5???
CO2 is polyatomic linear, so it shud have 3 translational and 2 rotational deg of freedom...
hey! see H C Verma .. wait i will tell the pg no.
yup.
part2 .. pg.71 .. 4th last line...
CH4, H20, CO2 all have six.
in some test, i did it with 5 and had got some negative marks i remember...
i too think it is 5 because linear...
but H C Verma can always be wrong [to err is humane]
so i also wanna know which is actually correct...
degree of freedom.....
transalational rotational vibrational
monoatomic 3 2 0
diatomic 3 2 1
triatomic (linear) 3 2 4 (CO2)
''' (bent) 3 3 3 (H2O)
this is frm ARIHANT......
can sum1 tell wats da rite values of degrees of freedom

well...i'm getting thoroughly confused..!! :p
this is frm wikipedia...according to this, deg of freedom for CO2 shud be 5...and at high temp, 9...
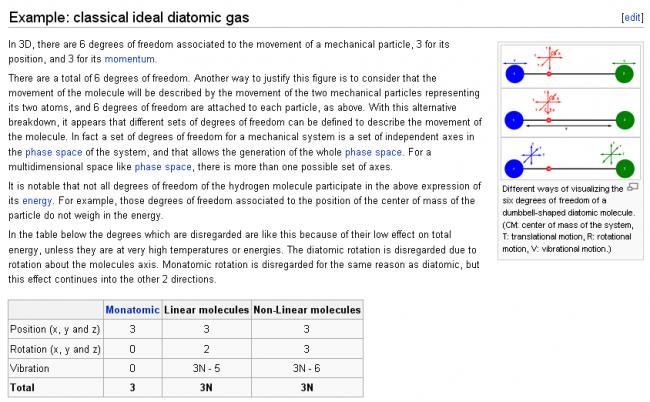
Let me clear it..............
Since CO2 is linear diatomic,so its vibrational motion will not be counted,........=>Degree of freedom=5
&&&&& since H2O or NH3 is non linear diatomic, so its vibrational motion too has to be taken care of..........=>Degree of freedom=6
arey yaar vibration sabko hoti hai... !!
lekin,,, only at higher temperature...
Whatever I have written up here is truth..................it comes to u all now whether u accept it or not.....[1][1][1]
no . all molecules vibrate but the energy associated is considerable only at high temperatures.
This is a very very controversial thing
You cant generalize for all gases
Srinaths's one line summarises it all.