is it a
The equation for the reaction in the figure below is
AB_{5}_{(g)} + heat\leftrightarrow AB_{3}_{(g)} +B_{2}_{(g)}
double arrow means the reaction is in equilibrium
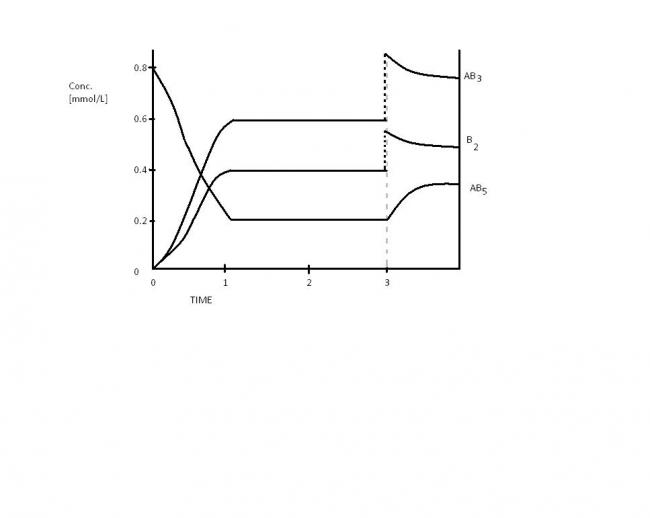
At\; time\; 3\; min\; , what\; change\; was\; imposed\; into\; the\; equilibrium ?? ?
[A] Pressure\; was\; increased\;
[B] Temperature\; was\; increased\;
[B] B_{2}\; was\; added\; to\; the\; system\;
[B] AB_{3}\; was\; added\; to\; the\; system\;
   Â
PS:only single ans is correct
-
UP 0 DOWN 0 2 29

29 Answers
It should be a,c,d. although i doubt the correctness of the question.
And aveek,a change in concentration of the reactants would certainly affect the equilibrium by shifting it in a particular direction(le chatelier's principle)
avishek it wud be so nice of u if u give ur ans wid a proper explanation [1]
Ya Aveek , I agree with you but , the graph shows the conc. of the products and reactants , not the eqlb. pt. What I think is , the reason is that simply adding the products won't affect the conc. because we have to decrease the heat content of the system so as to reverse the reaction , which is not given in the said options .
The simplest possible reasoning
Δn = 1
Hence now pressure being increased, the backward reaction will dominate....leading to the given curves.
Other options are faaltu and the most faaltu ones are C and D because increase in concentration of reactants or products doesn't change the equilibrium point.
The question now is how the conc. of thetwo products suddenly increase. Well I think this way : on increasing pressure volume decreases as a result of which conc. increases...as conc. varies inversely with volume.
And as for temperature ye ek natak hai.
See post#12
As mentioned by asish none is correct.
The condition given is true only if AB3 and B2 are added simultaneously
if the pressure of the reaction in a state of equilibrium be increased by keeping the temperature constant then the volume will decrease this means that the no of moles per unit volume will increase hence according to le chaterliers principle the equilibrium will shift in that direction which is accompanied by decrease in the number of moles per unit volume which is backward direction for this case hence the ANS are A , C, D
if this doesnt match with ur source guess what ur source is wrong!
C and D? For no change in the concentration of AB5, there's an increase in the concs. of the products. Since the reaction was already at equilibrium, the expected backward reaction predominates. Possibly to reduce the concs. of B2 and AB3. Um...and the answer is..?
all options cant be wrong ..... i will ask my professor abt this question today ...and will reply here
from the fig, if the graph is accurate then it seems that the value of Keq is not changing. So, temp change cant be a factor.
If B2 is added then [AB3] will decrease and not increase. So that is incorrect
Again if AB3 was added [B2] will decrease and not abruptly increase
If pressure is increased, the eqbm will shift in the direction where the no. of moles is less. So, [AB5] will increase. Further Pressure increases implies that [] of all components in the eqbm will initially increase.
So I would say all options are wrong.
see the graph at t=3
there is sudden jump in PCl3 and Cl2 concentration. But the concentration of PCl5 remains same
didnt get ur point sir....
i assume they are in gaseous state...so we consider their partial pressures...
pressure increases implies volume decrease: so the concentration increases
As we suddenly decrease the volume, concentration of all the molecules increases. But here concentration of PCl5 remains same.
Then how can (A) be true?
From graph,,the reaction is at equilibrium and change is made at eq due to which quantitiy AB5 increases and quantity AB3 and B2 decreases
Lets check all the options
a) Pressure increased => reactions shifts to left side
whch is true
b) Temp increased ,,, Since reaction is endothermic=> forward reaction shifts
which is false
c)B2 added => reaction shifts to left side
which is true
d)AB3 added => reaction shifts to left side
which is true
=> a,c,d
nope guyz ..its not a multiple correct ans ...... and the ans given is A.. ...