d
The pink violet colour ok KMnO4 is due to:
(a) d-d transition
(b) p-d transition
(c) charge transfer
(d) Both (a) & (c)
-
UP 0 DOWN 0 1 4

4 Answers
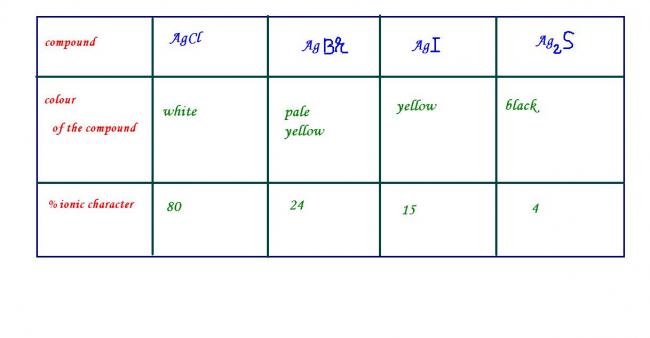
OXYSALTS of some of the transition elements ,e.g.KMnO4 and K2Cr2O7 are coloured though the central atom does not contain any unpaired electrons . the colour of thees compounds arises due to CHARGE TRANSFER SPECTRUM (colour is associated with the electrons being promoted from one energy level to another , and absorbing or emitting the energy difference between the two levels . thus transfer of an electron from M to L or vice versa results in charge transfer which gives rise to spectra .such spectra are called charge transfer spectra) . Ag+ does not contain any unpaired electrons but some of the compounds of silver are coloured which can be explained on the basis of ionic character . when the ionic character of these ionic compounds is less than 20% , then the compound will be coloured.