b
The Unit Cell of a crystalline solid is bounded by f (faces) , e (edges) and c (interfacial angles) . WHich of the following relation is correct:
A. f + e = c +2
B. f + c = e + 2
C. c + e = f + 2
D. NONE OF THESE
-
UP 0 DOWN 0 0 14

14 Answers
got it ! :P
one more ..
What is the maximum number of layers of atoms in closed packed planes that will lie within two imaginary parellel planes having a distance between them of 13 √2/3 R in the copper crystal (FCC) ?
COnsider the atoms to be within the parallel planes if their centres are on or within the two parellel planes.
Mere notes mein likha hai ki- Distance b/w A & B layers in a homogenous FCC packing(ABCABC..type)
= 2r√2/3.
So, tht way, ans shud be 6.....
Distance koi derive karo yaar, dimaag nahi chal rahaa...
yes tushar plz..post i also wanted the derivation for packing efficiency for hcp unit cell
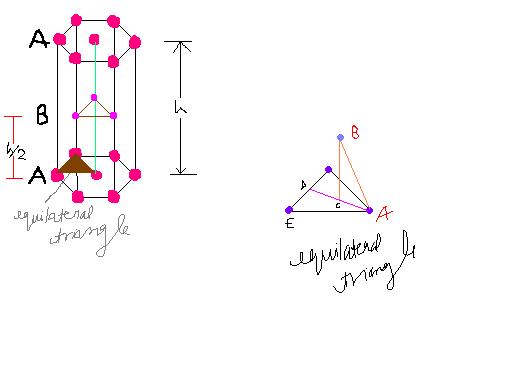
Layer A contians the corner atoms and the face centre atoms where as layer B contains within the unit cell
The distance b/w two succesive same layers is the height of the unit cell. All the layers are equivalent frm each other. All the atoms shuld be same. The corner atoms are touching each other along the edges of the regualr hexagon.
Since the regular hexagon can be divided into six equilateral triangles, it can be inferred that the corner atoms are making contact with the face centre atoms.
The atom of alyer B is located over the centroid of the equilateral triangle formed by three aotms of layer A. Atoms of layer B is making contact with all the three atoms of layer A.
AB = 2 r = AE
DE = R
In triangle DEA, DA= √(AE2 - DE2)
= √[(2r)2 - r 2 ]
= √3 r
Now, ACDC = 2
So, AC = (2/3) DA = (2/3) x √3 r = 2r / √3
Now, In triangle ABC, BC = h/2 = √[(AB)2- (AC)2]
= √[(2r)2 - (2r/√3) 2 ]
Therefore, h/2 = 2r √2/3
h = 4r √2/3
Derivitaion for packing fraction :-
V cell = (Area of base) X height
= (6 X Area of equilateral triangle) X h
=6 (√3a2 / 4) h
Putting h = 2a √2/3
= 3 √2 a 3
= 3√2 . 8 r 3
Therefore, V cell = 24 √2 r 3
V cell is dependent only on the radius of the atom,
Therefore, P.F = V atomV cell = 8 ∩ / (24 √2) = ∩ / 3√2
P.F = 0.74
Yeah, it does come down to 7 in the end, not 6.
The relation derived by tushar is fr an HCP (ABAB..) but works the same fr an ABCABC.. type stacking...!!
means the interplanar distance is same for ABAB... and ABCABC... type !!?