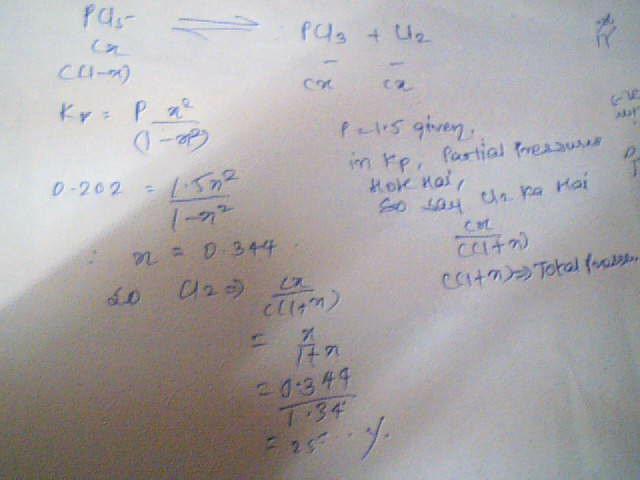ok..volume percentage has been changed to mole percentage
The volume percentage of Cl2 at equilibrium in the dissociation of PCl5 under a total pressure of 1.5 atm is? (Kp=0.202)
answer:25.5 %
-
UP 0 DOWN 0 0 8

8 Answers
Lokesh Verma
·2009-03-29 08:51:39
what does volume percentage means!
i thought that any gas occupies the whole volume and it is always 100% :O
sparkle2009
·2009-03-29 09:05:08
let me show you the solution given by the book which i didnt understand,may be that will give some idea
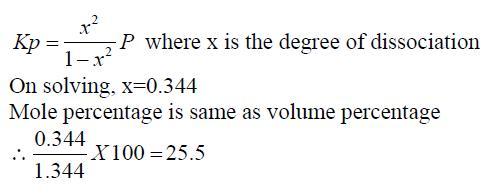
Lokesh Verma
·2009-03-29 09:11:01
if you assume that the reaction is taking place in gasseous state, then
take initial partial pressure as P
dissociation alpha
P(1-α) Pα Pα
then the thing you have done follows!
sparkle2009
·2009-03-29 09:12:05
lol new complication !
take is as a gas for time being..just show me how you solve those kind of problems !