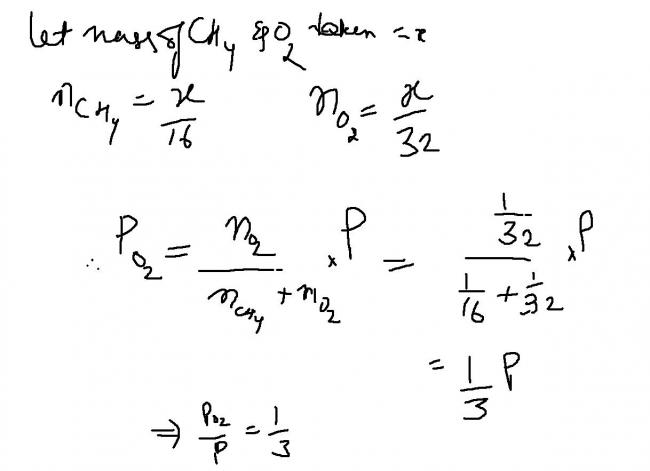Q2) answer is b
1) Equal weights of methane and oxygen are mixed in an empty container at 25 degree. The fraction of the total pressure exerted by oxygen?
a)1/3 b)1/2 c)2/3
2) At const vol. for a fixed no of moles, pressure of a gas increases wit inc in temp due to
a) Inc in avg molec. speed
b) Inc rate of collisions
c)inc. in molec attraction
d) Dec. in mean free path
3)In van der waal eq, the term which accounts for intermolecular forces is
a)v-b
b)rt
c)p+a/v2
d)rt-1
-
UP 0 DOWN 0 0 7

7 Answers
kamalendu ghosh
·2009-03-27 09:19:58
Pressure measured is the bombarment of molecules on the walls of a container