REASON AND MEHANISM OF HYDROGEN BRIDGE BOND IN THE DIMER , BORON TRI-HYDRIDE MOLECULE.
-
UP 0 DOWN 0 0 2

2 Answers
BH3 always exist in dimer state forming B2H6
whose structure follows as: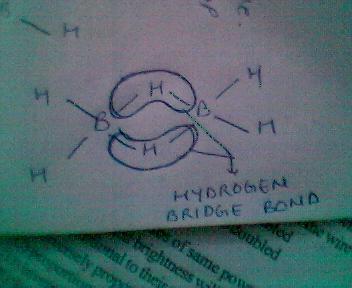
this completes the incomplete octet of Boron and is also known as two centred three electron bond or banana bond!!!!!!
similar bond also exist in AlCl3 where also it exist in dimer form!!!!!!!!!!
cheers!!!!!!!!!!
two centred three electron bond......three centered two electron bond
and case of AlCl3 is different AlCl3 uses pπ-dπ bonding also known as back bonding for its dimer structure
basically three centered two electron bond formed by hydrogen or methyl or phenyl groups as middle specie and stability order is
hydrogen > phenyl> methyl
but i dont think u will be needing this stuff
tell me if u want more details