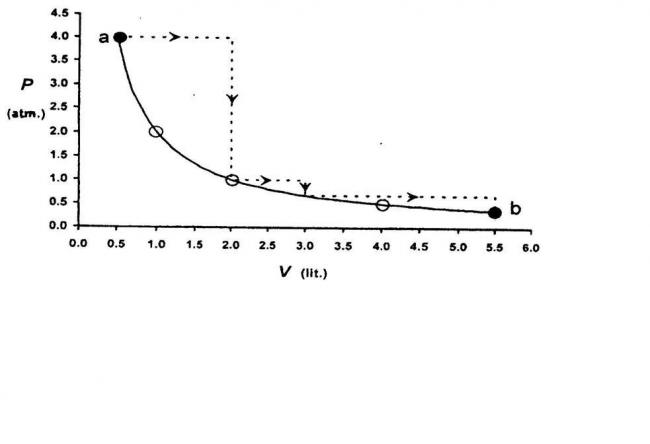
the dark line represents isothermal process
and the dashed line consists of combination of sobaric and isochoric processes
use the relevant equations to find the work done in each case....
how to find the work done in the two processes?
plz help
-
UP 0 DOWN 0 0 4

4 Answers
AKHIL
·2011-03-14 11:03:22