pH of an acidic buffer is given by
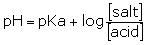
here [salt] =0.1M
[acid]= 0.1M
so pH will be = 6
the pH of a solution is 0.1 M NaA and 0.1M HA ( Ka = 1 x 10^-6) would be :
a)5 b)6 c) 3 d) 7
detailed answer shall be appreciated .......
i cannot understand the question...may be something wrong in the language...please post it clearly !
I have not analysed much but i think the pH will depend on the quantity of HA and NaA added (u need the volumes of both)
pH of the mixture of a weak acid and strong salt is
pH = 7+ pKa/2 +(log c)/2 .......ka is for the weak acid and (c) is the concentration of the weak acid !
pH of an acidic buffer is given by
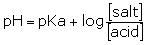
here [salt] =0.1M
[acid]= 0.1M
so pH will be = 6
oh yeah...i could not figure out that this one is a buffer ! missed out this sitter ...shit ! [2] [2]