maybe c tooo
becoz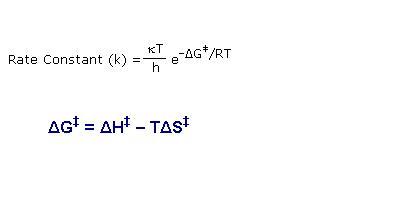
According to transition state theory, rate constant (K) will depend on
1. Temperature
2. Threshold energy
3. Entropy change from reactant to transition state
4. Collision frequency
-
UP 0 DOWN 0 0 6

6 Answers
roboarmy filas
·2009-12-28 21:31:24
we know k = Ae-Ea/RT.
where A is collision number.
thus from the equation it depands on temparature,collision frequency and activation energy(threshold energy).
Asish Mahapatra
·2009-12-31 01:01:43
ans was given abc .. (which was wrong uttara)
it is indeed abcd..
our sir also said so