Q1
http://targetiit.com/iit-jee-forum/posts/only-for-admins-kinetics-bhooooooooooomm-4628.html
Q1. For the reaction 2HI --> H2+I2 the rate constant is k. What is the rate constant of the reaction HI --> H2/2+I2/2 ?
Q2. Draw the graph of the temperature dependence of the rate of reaction R of a simple reaction. i.e. draw the graph of R vs T
-
UP 0 DOWN 0 0 10

10 Answers
thx eure but i hav a dbt.
isnt the rate constant of a reaction constant for any stoichiometric coefficient? although i understand wht u hav rwitten and why i still have dis dbt that y the rate const will not be const
Q2
let reaction A→B
k=Ze-E/RT
also rate of reaction=k[A]m
=> R=Ze-E/RT[A]m
=> R=λe-1/T
i know can u give a general shape? i needed tha answer for RCM's objective question 50? the shapes are given i just needed the shape
Q2
Since T is absolute temp => T>0 always => graph in 1st quad only
diff wrt T
=> R' =λe-1/T/T2 >0 for all T>0
=> R is increasing
and R' ≠0 for any T
Taking second derivative => R" <0 for all T >0 => convex shape
As T→∞
=> e-1/T→1
=> Max value of R=λ
So maybe graph can be :::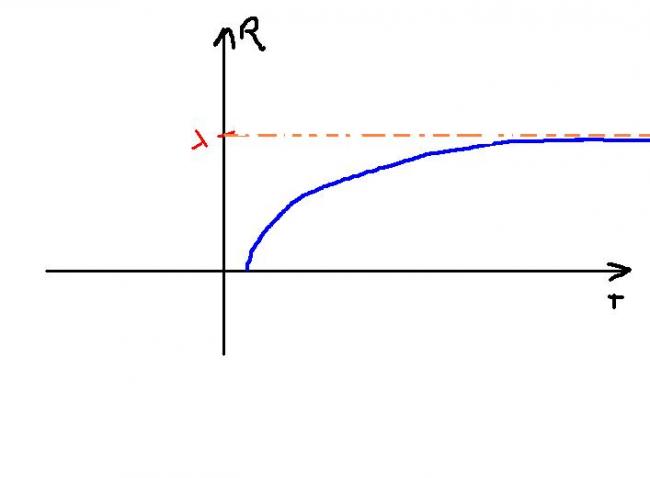
Plz tell me if there is any error in working
i also think the same but in RCM the graphs shape was given concave upwards i.e. dy/dx as well as second derivative positive
and i think book may be wrong ..becoz if u and me are getiing same anss..then i think its right