its an 2008 question
but BEWARE to post the solution
I HAVE A LOT OF THINGS TO ASK
Q
2.5 mL of 2/5M weak mono acidic base (Kb = 1 × 10–12 at 25°C) is titrated with 2/15 M HCl in water at 25°C. The concentration of H+ at equivalence point is (Kw = 1 × 10–14 at 25°C)
(A) 3.7 × 10–13 M
(B) 3.2 × 10–7 M
(C) 3.2 × 10–2 M
(D) 2.7 × 10–2 M
its an 2008 question
but BEWARE to post the solution
I HAVE A LOT OF THINGS TO ASK
nahin yaar yeh to abhi net se download mara hai
mere pass doosre solution pade hain
uss mein
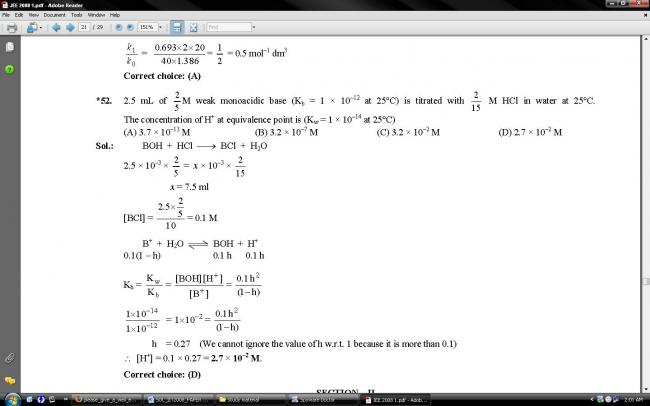
2.5 mL 2/5 M base is neutralized by 15/2 mL of 2/15M acid
Salt concentration = 1/10
C = 0.1
Kw/Kb = Ch2/1-h
On solving, h = 0.27
[H+] = Ch = 2.7 X 10 2 M
10h2+h-1=0
h=(-1+√41)/20=0.27
ch=[H+]=0.1*0.27=2.7*10-2
mani u just find out concentration of the salt at the equivalence point.
then u use the formula ph=.5(pkw-pkb-logc)
actually this is JEE 2008 question
I HAVE THE SOLUTION WITH ME BUT STILL I AM NOT CONVINCED
bhai ........................
abhi the question and the options r perfect!!!!!!!!!!!!!