1..
plz balance the following equations:
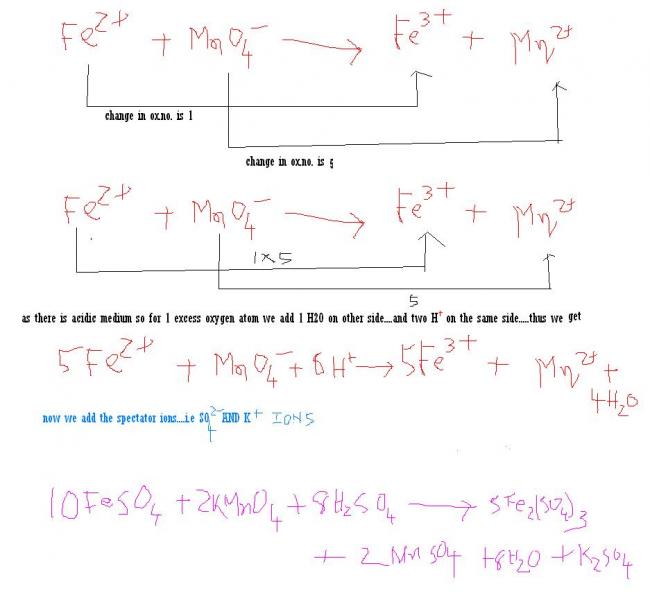
1)KMnO4 + FeSO4 + H2SO4->K2SO4 +MnSO4 + +Fe2(So4)3 + H2O [ by oxidation no. method ]
2) Cr2O7 + NO2- -> Cr3+ + NO3- [by half reaction method]
-
UP 0 DOWN 0 0 3

3 Answers
Cr2O7 +NO2- - -> Cr3+ +NO3- (Neutral medium)
Let us take first part:
Cr2O7 →Cr3+
Cr2O7 →2Cr3+ (Balancing chromium on both sides)
Cr2O7 →2Cr3++7H2O (7 water molecule added to oxygen deficient side)
Cr2O7 +14H+ →2Cr3++7H2O (to balance hydrogen-14 H+added to hydrogen deficient side)
Cr2O7 +14H++8e- →2Cr3++7H2O .....(1) (to balance charges 14+ & 6+, 8 e- is added to LHS)
Now let's take the 2nd part:
NO2- →NO3-
NO2-+H2O →NO3- (1 water molecule added to oxygen deficient side )
NO2-+H2O →NO3- +2H+ (to balance hydrogen-2 H+added to hydrogen deficient side)
NO2-+H2O -2e- →NO3- +2H+ ......(2) (to balance charges 1- & 1+, 2 e- is subtracted from LHS)
To equalize the number of electrons gained by (1)st part, the (2)nd part must be multiplied by 4, so (2) becomes
4NO2-+4H2O -8e- →4NO3- +8H+
Now a solution of simultaneous linear equation between (1) & (2) :-
Cr2O7 +14H++8e- →2Cr3++7H2O
4NO2-+4H2O -8e- →4NO3- +8H+
On adding (1) & (2) :-
On cancelling the electrons-
Cr2O7 +14H+ →2Cr3++7H2O
4NO2-+4H2O →4NO3- +8H+
On cancelling H2O molecules-
Cr2O7 +14H+ →2Cr3++3H2O
4NO2- →4NO3- +8H+
On cancelling the H+ ions-
Cr2O7 +6H+ →2Cr3++3H2O
4NO2- →4NO3-
Final addition of reactants & products:-
Cr2O7 +6H+ + 4NO2-→2Cr3++3H2O + 4NO3- (The equation is balanced and charge on both sides are equal that is 2+)