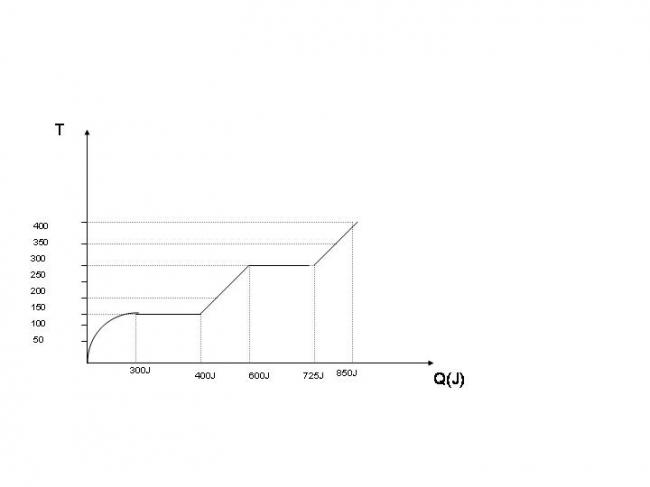
LAtent heat of fusion = L
Q = mL
100 = 5*l
L = 20J/g
L = 20000J/Kg
Latent heat of evapouration = N
Q = mN
125 = 5 *N
N = 25J/g
N = 25000J/Kg
Moles = 5/50 = 0.1moles
For temperature before liquid C(Molar Specific Heat)
Q = n*C*T
300 = 0.1 * C *150
C = 20J/moleK
For Liquid
Q = n * C *T
200 = 0.1 * C * 150
C = 13.3J/moleK
For Gas
Q = n*C*T
125 = 0.1 * C *100
C =12.5
Average = 20+13.3+12.5/3
C(average)=15.26J/moleK