lone pair electrons always exist in pairs because they have opposite spins and hence cancel the effect of each other......
i hope i am right....
13 Answers
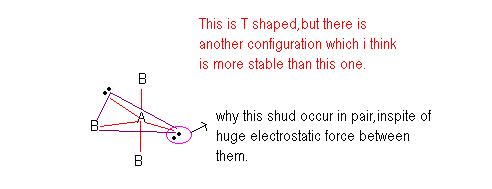
Whats the more stable configuration according to you?
Also an electron always form a pair in an orbital if they there is another electron available.
y it shud form a pair.if it exists in pairs kq2/r2 r is so small, so huge force.so i think there must be some other reason,wat is gong to be changed incase of its spin.
can u explain breifly.
opposite spins....so opposite electric fields.....hence they cancel out....
net electric field=0.....
in ur structure...there are 3 90° bonds between l.p and b.p.
which results in unstable structure....as far as i know.....
yeah this is wat i thot,but we dont know the relative stability,i want more confirmations da.
@msp. Dude. Your structure is unstable according to me coz your structure has each electron pair has to face more repulsion from 3 bond pairs which are at right angles to it. U have placed lone pairs away but 3 lp bp repulsion is more than that. It increases the energy of the system and hence makes it unstable.
just like we will do in finding the structure which contributes in the resonance hybrid.
Just find the number of repulsions to arrive at the right structure
lone pair=LP
bond pair=BP
the order is LP-LP>LP-BP>BP-BP
ya,eure is right.this is the right way of judging the relative stability of structures.
the order LP-LP>LP-BP>BP-BP is considered effective only when any of these pair is at 900 wid each other.repul;sion is considered negligible when angle is 1200 or 1800. also, neglect the BP-BP repulsion as its too weak compared to the other two.
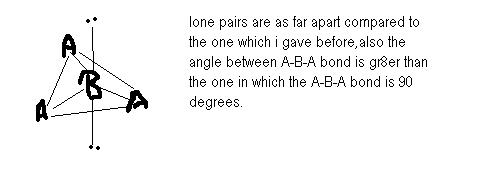
in the first structure : there are four 900 repulsions between lonepair and bonded pair and no lonepair-lonepair repulsion.
in the 2nd structure there are six 900 repulsions b/w bonded pair and lone pair but no lonepair-lonepair repulsion.
thus, 1st structure is more stable then the 2nd one.