29
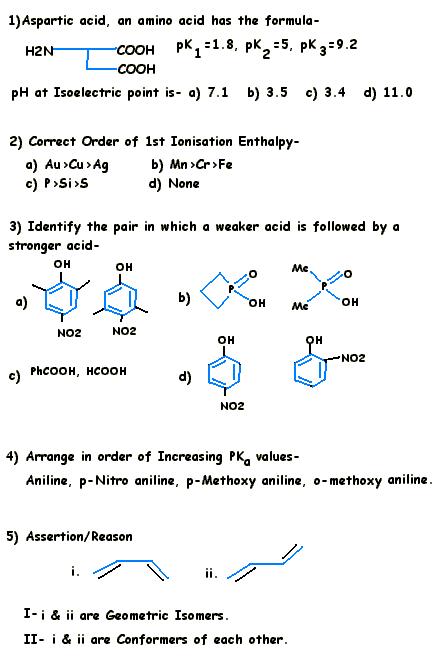
29Ans 1 ...it shud be NH3+ ..anyways Pka of isoelectronic point in such question are calculated by seeing the average value of pka1 and pka2
where pka1 is the pka wen the total charge on the compound changes from +1 to 0
and pka2 is the pka wen the charge on the compound changes from 0 to -1....
so (1.8 + 5)/2 = 3.4
Ans 2 A
Ans 3 C
 29
29Ans 3 is c definitely....that's an exception..in d i think u missed the hydrogen bonding funda...second structure in option d will be less acidic due to hydrogen bonding
 1
14.)p-nitro aniline < p-methoxy aniline < o-methoxy anline < Aniline
 13
132) a) rite....is this a thing to be learnt ?
3) c) rite...But koi je batao ki why isn't (a) correct too...is smthing like Ortho effect working here ?
4) Yup, avinav's rite...But kaise aaya ?
5) d) was the ans given, but how can they be Conformers..(Rotation is Hindered!,,,sp2 Hyb.) ?
 106
1061. (1.8+5)/2 = 3.4
2. It is due to lanthanide contraction.. (although i still dont know what to do abt order Au>Ag but not sure if Au>Cu also)
3. in (a) due to steric effect.. the OH grp goes out of plane so, delocalisation due to methyl groups is absent.. Hence it becomes more acidic.
4. ortho effect..
o-methoxy is more acidic than aniline..
(i think p-methoxy will be first though)
5. rotation is not taht much hindered in this case.. only abt 4kcal/mole.. hence they are conformers.
 1
12 a WHY?
3 c,d (govind's expln is correct) o-nitro phenol is a stronger acid will lose H+ and form hydrogen bond after losing it. p-nitro phenol has intermolecular hydrogen bonding. 4 d (assertion is false) rotation is not hindered they are rotating about single bond.
 29
29@Tapas...o-nitro phenol is a weaker acid as compared to p-nitro phenol..
 1
1why?
reference:
fiitjee ft 1 2010 chem paper 2 q12
they have stated p-nitrophenol is a weaker acid than o-nitrophenol
 13
13O-nitro phenol mein Intramol. H-bonding hai, thts why weaker.
1) btw, Wht is Isoelectric point, haven't done this sort of Qn b4.
4) I too thought p-methoxy should have been the 1st one.