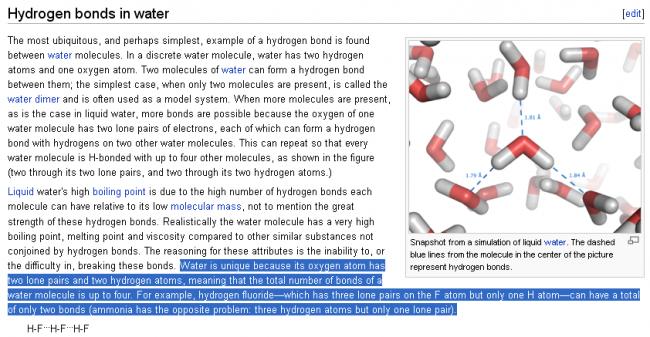
11
11Shreyan, pls see carefully......in HF, the total no. of bonds is two (one is H-F and the other is H...F)
I am not sure about water.......with the same logic as above, water should have total of 4 bonds....... H-O (2 bonds) and H....O (2 bonds)
THIS ANSWER HAS 0 PROBABILITY OF BEING CORRECT [4]
 1
1it is not zero it is near 2 correct answer but real answer is get when we ask water molecule how much he had form ?? hehehe par for us only 4h-bond by H2O and 2 by HF see ani. photo mast hai yaar i thinking it is not u ani
 1
1
ye kuch water ki tarah dikhta hai.........:)
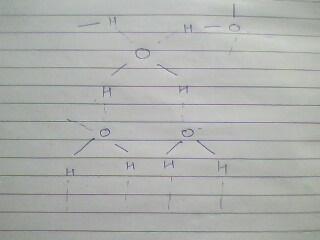
aur ye, uske char H- bonds
 1
1H-F ka exact structure dekhne ke lie refer to test section.......:( i dont remember test no