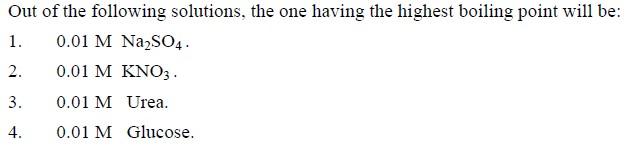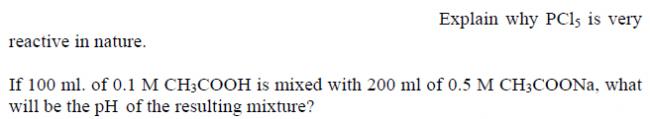29
29Ans 1..acidic..cationic
Ans 2..Na2SO4 ...van't hoff factor is 3 in this case..
Ans 4..PCl5 is very reactive coz it has two kinda bonds..one is equatorial bonds which are short and stable and the other axial bonds which are long and relatively unstable..so those two axial bonds can be easily broken....thus making it reactive
 1
11) agreed
2) agreed
4) "axial bonds can be easily broken" reason?
 39
39Q2. Van't hoff factor for sodium sulphate will be i = 3 as two sodium ions and one sulphate ion are formed on dissociation. So it's molar concentration becomes 0.01 x 3 = 0.03 molar. Next in line is potassium nitrate with factor 2 so it's molar conc = 0.02 molar. So decreasing order of elevation in BP should be -:
Na2SO4 > KNO3 > Glucose > Urea (Last two decided on basis of mols in their 0.01 molar solutions, glucose has more mols?)
Axial bonds in PCl5 are highly strained and hence longer than the equitorial bonds. Thus it loses a chlorine molecule by heat and becomes PCl3.
 1
12) agreed
4) thx
3) \Delta H^{o}_{f} \; H_{2} required??? thts wat i feel!
 29
29Ans 3... C + O2 → CO2 ....1
C + 1/2O2 → CO .....2
H2 + 1/2O2 → H2O .....3
Now on adding 2 and 3 ...and subtracting 1 ..u will get..the required equation..
so answer will be : (-110.5 -244.8 + 393.5)KJ
Heat of formation of compounds in their standard state is 0..like H2, O2, N2 etc..
Ans5 .. use that formula pH = pKa + Log( [Salt] / [Acid] ) ..for buffer solution
 1
13) thx for clearing misconception
5) no pka available???
 29
29well that is 1.8 * 10-5 ...but it shud be mentioned in the question...
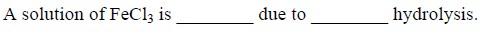 (acidic/basic;cationic/anionic)
(acidic/basic;cationic/anionic)