GREAT PRITISH.....TRY Q1.
1.The hydride which does not react with water
a)NH3 b)PH3 c)DIBORANE d)AsH3
2.WHICH ONE IS THE STRONGEST BASE?
a)OH- B)HS- c)HSe- d)HTe-
3.GIVE PRODUCT(S).
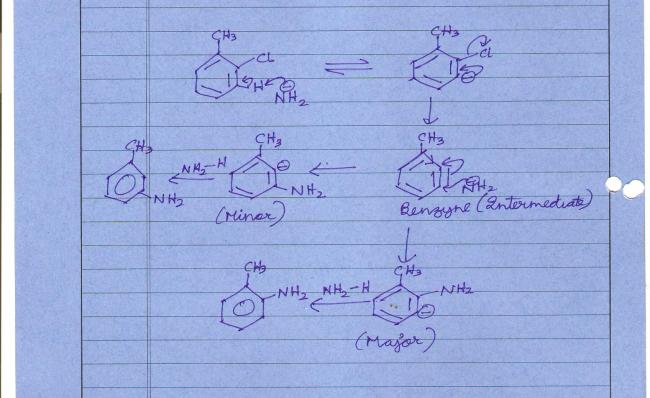
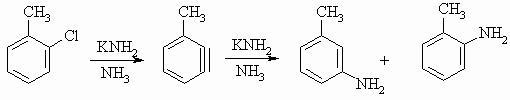
otho-chloro-toluene..REACTS IN PRESENCE OF ..sodamide and liq.ammonia..
-
UP 0 DOWN 0 2 14

14 Answers
2. Compare their conjugate acids' strength.
H2O < H2S < H2Se < H2Te
This is because atomic size increases down the group, due to which bond dissociation enthalpy decreases and it becomes easier to give up a proton. Thus hydroxide ion is strongest base(basicity follows reverse order).
3. Benzyne mechanism question. In the benzyne mechanism R-effect is not considered, only inductive effect. Minor and major products hain kyunki methyl group ka inductive effect us anion ko destabilise kar deta hai.
Also starting step mein the beta hydrogen which is more acidic is abstracted by amide anion...again due to methyl inductive effect.
1. Phosphine, or PH3, I think. The P-H bond is not so polar.
@Pritish...can u plz explain why m-methylaniline is minor product and o-methylaniline is major product...coz i think it shud be the other way....
Moreover see this thread http://targetiit.com/iit-jee-forum/posts/benzyne-mechanism-13672.html
the reason why i feel that m-methylaniline is major and o-methylaniline is minor is that CH3 is an ortho-para directing grp..so it shud increase the electron density at the ortho and para positon ..since NH2- is a nucleohile so it wont attack at the ortho position..Moreover Robert Thornton Morrison and Robert Neilson Boyd are also saying that Meta product shud be major...[1]
and phoshine has vacant orbitals wer it accept the lone pair from Oxygen
and we know the complex NH4oh formed with rxmn with water
so be elimination d is right
WHEN CHLORINE IS ATTACHED TO METHL GRP...AT ORTHO POSN.....NH2..WILL B ATTACHD IN THE ORTHO POSITION....BCOZ..THE CARBANION PRODUCED WILL B MORE STABLE....THAN CARBANION FORMD..WEN NH2 IS AT META
Edit : Understood...you want to say that the carbanion being at meta position is less affected by ortho para direction of methyl group. Ortho-para direction applies outside EAS?
One more thing...inductive/polar effect acts like resonance? Resonance cannot be applied in benzyne mechanism. I think inductive effects act only on the carbons near to them...they do not suck or give electrons as a whole and leave meta position intact like it happens in resonance.
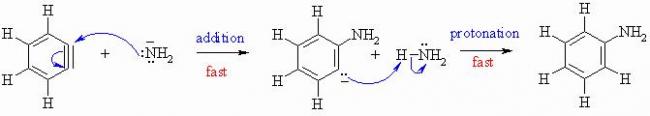
since the addition of NH2- is fast WILL THERE BE A MAJOR PRODUCT?
If yes then, META NH2 addition.
PH3:
It is highly reactive. PH3 unlike NH3 is not very soluble in water; aqueous solutions are neutral.
P-H bond energy: 318 kJ/mol
AsH3 :
The bond energy and the stability of hydrides both decrease on descending the group. Consequently, arsine is obtainable in small amounts only.
As-H bond energy: 247 kJ/mol
I have doubt b/w option B and D?
Please note that the major and minor products in post #3 are wrong from my side. I will explain now how the mechanism of this reaction actually works -:
Step I : The most acidic beta-hydrogen(beta to Cl or leaving group) is abstracted. Methyl group's +I effect makes the beta-H it is acting on less acidic. So as I showed in the diagram in post #3, the other proton is abstracted. Everything's fine here.
Step II : Benzyne is formed and -Cl is knocked out. Yahan tak sab kuch theek hai.
Step III : And now we come to the nucleophile's attack step. The nucleophile will attack the position of least electron density, and deciding by the methyl group's inductive effect, the farther away from methyl the site of attack is, the better for the nucleophile. This is the RDS of the reaction! Hence if the nucleophile attacks a position of less electron density, the reaction proceeds faster and is more feasible. It is the RDS we consider, and not the stability of the carbanion as I have mistakenly mentioned earlier.
Step IV : A fast acid-base reaction gets rid of the carbanion, irrespective of how "stable" it was or not.
Going by these steps, it is meta methylaniline which is the major product and o-methylaniline the minor product. This is because the RDS proceeds quicker when NH2- attacks the meta position due to +I effect of -CH3.