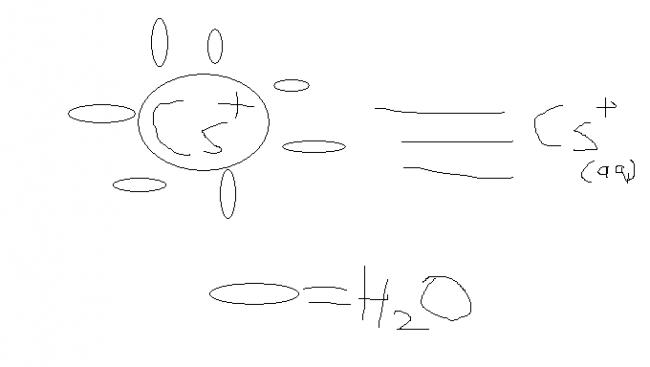
when ions in aqueous state(medium) the medium molecules attached electrostatically to the ion it is invesely proportional to size and directly to charge below figure can help..
WHAT DO U MEAN BY SOLVATION OF IONS(EXACTLY)???????????
-
UP 0 DOWN 0 0 6

6 Answers
solvation and hydration are two diff concepts...i think u r getting confused in both...
what i think is that u want to ask about hydration of ions...
actually the concept of hydration is that when a compound(ionic) is dissolved in water, it gets dissoiated into ions...these ions interact with water molecules...know how???
the cation is positively charged and water has 2 lone pair of e-s, . smaller the size of the cation, more the water molecules will b there surrounding it...
this hydration depends on 2 factors, one is lattice energy and the other is hydration energy...ye bhul gaya lattice energy kum honi chahiye ya zyada...but i think kum honi chahie...
so, the factors lattice enrgy(less) and hydration energy(high) favour the hydration...
this was my concept about the whole thing...i hope it is helpful..if find ny doubt or mistake, plz ask...
and 1 thing i wud mention again that i dont think that solvation and hydration r similar..
bahut zyada likha..huh...thak gaya...pehli baar, itna bada post...:p