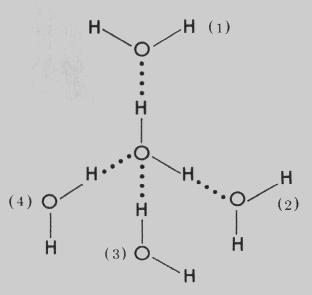
H2O has hydrogen bonding in between molecules and each each H2O is attached to 4 other H2O molecules with Hydrogen Bonding.
Hence the intermolecular forces are stronger and water has a high BP.
H2S does not possess hydrogen bonds....
So it can boil at lower temperatures than water.