can u explain why adding an electron to just 1s2 config easier than adding an electron to 1s22s22p6 config?
Can anyone plz explain the reason behind the trend of electron gain enthalpy in the following table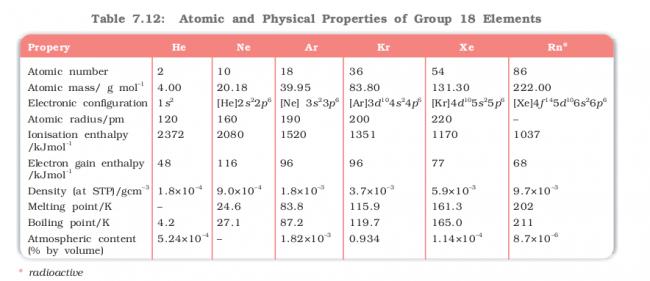
btw in the above table there is a mistake in the atmospheric content row the -- should in case of Radon instead of Neon
-
UP 0 DOWN 0 1 11

11 Answers
We know electron gain enthalpy is positive because of completely filled subshells. We have to supply energy to add an electron.
Going down the group, size increases, and electron gain enthalpy becomes less positive.
If we compare just neon and helium, this is obviously an anomaly....is adding an electron to just 1s2 config easier than adding an electron to 1s22s22p6 config? That would explain the anomaly.
Yes I think I can....we know that s < p < d < f in terms of distances of orbitals from the nucleus. s orbital being closer to the nucleus, has its electrons strongly attracted to by the nucleus. There is not much repulsion for the new electron coming in. In neon's config, the s-orbitals already shield the nucleus to a certain extent, and p-orbital electrons repel the electron being added greatly.
u may download that question bank if u want just search KV Hots in google ..u will get the link.....
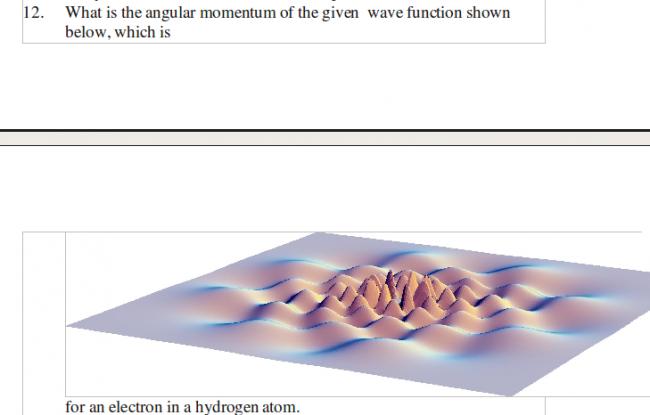
ans given at the back is n=8..Reason....If we trace a circle going around the center, we run into a series of eight complete Wavelengths.