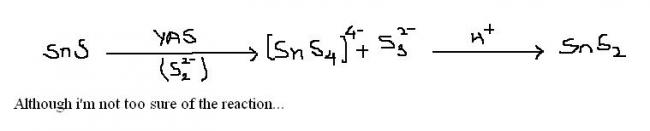Q1 what happens when stannous sulfide treated with yellow ammonium sulfide ??
Q2 How can Co and NI be separated
Q3 How can Cu2+ and Cd2+ be separated ??
-
UP 0 DOWN 0 0 4

4 Answers
Shreyan
·2009-11-12 10:10:43
Q1 first stannous sulfide dissolves...then on addition of H+, it forms stannic sulfide.
govind
·2010-02-22 01:34:15
1. SnS is solube in (NH4)2S ..the reaction given by shreyan is right..
2. Ni and Co can be separated by DMG(dimethyl glyoxime) Ni forms red ppt with DMG..
3. Cu2+ and Cd2+ can be separated by K4[Fe(CN)6]...Copper forms a chocolate brown ppt..with K4[Fe(CN)6]