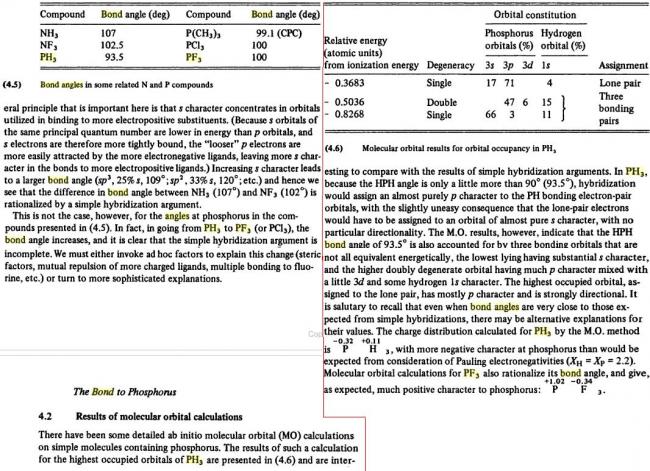
NH3>NF3>PH3>PF3
Arange the following in order of dec bond angle
NF3,PF3,NH3,PH3
-
UP 0 DOWN 0 1 11

11 Answers
NH3 > NF3 > PF3 >PH3
as PH3 is highly unstable comp. as it has bond angle =91°
@ xyz drago's rule is applicable . so smalll -ve charge is on P in PH3
@kartick jus now i checked with wiki.........
u r right.....[6].........
can u explain the drago's rule
drago's rule is applicable on atoms which are less electronegative & there exist a small -ve charge on it which unstablize the central atom
thus dec. it's bond angle to approx. around 90°
some ex. H2S, PH3
this cn be analysed without using drago's rule also.
Comparing NF3 and PF3 , bond angle of NF3 > PF3 as N is more electronegative thn P ; thus N 's nucleus attracts bonded pairs more thn P. thus , the distance b/w bonded electrons is less in N, which increases its bond angle.
Here, P hs a lone pair but its effect is negligible. this is becoz, the lone pair is equitorial to 2 P - F bonds and 900 repulsion to a P - F bond , but the bonded pair remains shifted towards F , thus ders minimum effect. but this effect is a bit more in NF3 , as N is more e-ve thn P and bonded electrons remain less shifted towards F in it.
now comparing NF3 and NH3 , bond angle of NF3 < NH3 .
here, the lone pair effect is dominant. In NH3 , 900 repulsion b/w lone pair and axial N - H bond and here bonded e-s remain shifted towards N. This effect is not available in NF3.
same effect with PF3 and PH3
Now, among NF3 and PH3 , again the lone pair - bond pair repulsion comes into account, which would be dominant in PH3 as the electrons remain shifted towards P.
thus , bond angle of NF3 < PH3 .
thus, finally bond angle order is : NH3 > PH3 > NF3 > PF3
this is what should come without using drago's rule !
@ kartick , in # 4 , whats the link of an unstable compound wid its bond angle ?
might be. bt , i don't think, i hv analysed out smthing wrong, unless , ders some real exceptional case hidden in the question.
ans is correct
see this:
http://books.google.com/books?id=YEE5AAAAIAAJ&pg=PA27&lpg=PA27&dq=bond+angles+of+PH3+and+PF3&source=bl&ots=9RBmOkdn7S&sig=hSu13mD3wM0FD1W-8g1AU-aghI0&hl=en&ei=H-_3SvbsF5P6sgOUv6gS&sa=X&oi=book_result&ct=result&resnum=2&ved=0CAoQ6AEwAQ#v=onepage&q=bond%20angles%20of%20PH3%20and%20PF3&f=false
reason for PF3 and PH3 here the bonding involves more of p character rather than sp3 character.
And in NF3 and NH3 there is more sp3 character.
So, ans is
NH3>NF3>PF3>PH3