2. Two bubbles of radii r1 and r2 are connected.
(i) In which direction will the air molecules move?
(ii) What will happen to the size of the bubbles?
Please post complete solution
1. Consider two bubbles of radii r1 and r2 respectively. Let the are allowed to coalesce, Find the common radius of the interface
2. Two bubbles of radii r1 and r2 are connected.
(i) In which direction will the air molecules move?
(ii) What will happen to the size of the bubbles?
Please post complete solution
2.) the one with smaller radius will become smaller n the one with larger radius will become even larger...
This is wat i think
Let r1>r2
Then excess pressure inside 1 is less than in 2. (P=4T/r)
So, air molecules will move from 2 to 1.
Hence the pressure inside 2 will decrease and inside 1 will increase.
Hence the size of 2 will increase and 1 will decrease (P=4T/r)
well .... i have no reasons...
i would have given u the same ans...
but that is wrong...
this was the explanation of both sirs.... i am going to ask the third sir tomorrow........and see what he has to say
:)
am sorry ashish...
but thats the ans for sure....
hope some one explains....
it has been my doubt as well...
@asish
The explanation given by the sirs is absolutely correct.Let me give you another example : Consider two vessels of different capacities,containing the same no. of moles of the same gas at the same temp.This obviously tells you that the pressures are different.So,when the stopper is removed,should there be flow of gas??or do you say no flow of gas because the no. of moles is the same??Actually,there will be a flow of gas from the left vessel to the right vessel such that finally,pressures in both the vessels are the same (and NOT no. of moles)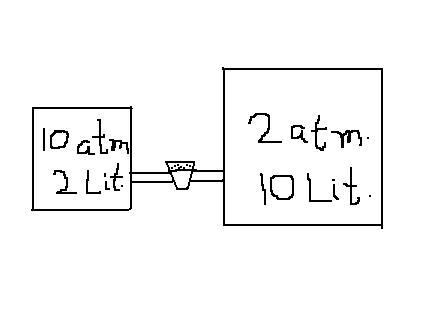
So,in your Q2) also air molecules will flow from bubble with higher internal pressure to that of lower pressure,i.e.from smaller bubble to bigger,since internal press. is inversely prop. to radius.
Subsequently,as the press.inside the smaller bubble decreases,its size will increase and vice versa in the bigger bubble!!
The second law of thermodynamics also explains the explanation given by asish
but i havnt given any explanatin!
its my doubt as well .....
can u explain pls...
air flows from higher to lower.... hence smaller bobble (with HIGHER P INSIDE) WILL SHRINK AND VANISH AS AND WHEN CONNECTED TO A LARGER BUBBLE.
THIS WAS SIMILAR TO 1 OF QUES IN JEE 2K8